(b) Determine the empirical Two substances have the same molecular and empirical formulas. 1,2-DICHLOROBENZENE (O-DICHLOROBENZENE) (SEE ALSO: 1,3-DICHLOROBENZENE (541-73-1) & 1,4-DICHLOROBENZENE (106-46-7)), 1,2-Dichlorobenzene solution, certified reference material, 5000 mug/mL in methanol, 1,2-Dichlorobenzene solution, certified reference material, 200 mug/mL in methanol, 1,2-Dichlorobenzene, PESTANAL(R), analytical standard, o-Dichlorobenzene [UN1591] [Keep away from food], o-Dichlorobenzene [UN1591] [Keep away from food], 1,2-Dichlorobenzene, HPLC, 98.0% min. For example water. Polar molecules arise when there is an electronegativity difference between the bonded atoms. If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. CN2 This is how many actual elements of each type that are present within that compound. Where is the magnetic force the greatest on a magnet. WebC6H4Cl2 The chemical formula of 1,2-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. We're going to say this example question, it says determined the empirical formula off a compound that is 68.40% chromium and 31.60% oxygen. *Response times may vary by subject and question complexity. Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox. So step one, you're going to write down the symbols for each element in the question we're dealing with chromium and its symbol a CR and oxygen with its simple as just Oh, now, Step two. How many credits do you need to graduate with a doctoral degree? Convert all the masses into moles. like to publish our findings in the Journal of Organic Chemistry. be CH2O.). C6 H12 O6 =CH3O = 1:3:1. what is the empirical formula of glucose. They don't share any number in common where we can reduce it to a simplified form. Here we have the molecular formulas of different compounds. Given the molecular formulas, write the empirical formulas Textbook Question. P4O10 WebSo here we are provided with the compound c, 6 h, 4 c, l 2. EC No. 1,2-Dichlorobenzene solution, NMR reference standard, 1% in acetone-d6 (99.9 atom % D), NMR tube size 3 mm x 8 in. -. Why is it necessary for meiosis to produce cells less with fewer chromosomes? carbon and hydrogen. So that would mean that the empirical formula here is CR two 03 So this would be my empirical formula for this question, and these are the steps you have to employ in order to get the empirical formula when given information on the percentages or mass of different elements within it, Additional resources for Empirical Formula. WebWrite the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6, (b) C8H10, (c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. amu answered. Let us suppose that we have just created a new procedure to make glucose, the simplest of all sugars, and we'd Need to identify active compounds in your natural products?  If the formulae agree, then our sample may be benzene. We dont have your requested question, but here is a suggested video that might help. UnitPot is a noteworthy web-based scientific unit converter that comes with an intuitive user interface. 1) CH, NaS04 For physicochemical, thermodynamic, transport, spectra, and other property data & information, the followings are available from Mol-Instincts, a chemical database based on quantum chemical computations: The structure data file (SDF/MOL File) of 1,4-DICHLOROBENZENE is available for download in the SDF page of 1,4-DICHLOROBENZENE providing the information about the atoms, bonds, connectivity and coordinates of 1,4-DICHLOROBENZENE, which is not completely available in the chemical formula representation. The compound 1-propanethiol, which is the eye irritant released when fresh onions are chopped up, has the chemical formula C3HyS and a formula mass of 76.18 amu. Q:4. a. Molecular mass is the sum of masses of all the elements present in the molecule, expressed in, Q:What is the correct name of the compound, HCIO2? Is Brooke shields related to willow shields? Q:How many moles of NF3 contain 2.55 x 1024 fluorine atoms? Structure Search. (a)rubidium nitrate,RbNO3, A:Following are the formula mass of the given compounds. CuSO4 is an empirical formula. Advanced Search. What is a dual sport motorcycle used for? If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. The following diagram represents the collection of elements NaHCO,: The 1,4-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. If the formulae agree, then our sample may be benzene. It's best to have at least four decimal places. the following molecular formulas: (c) C4H8O2, Write the empirical formula corresponding to each of C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid. B. O 156.26, A:4. should be if we really made glucose. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. 3. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C6H4Cl2 C6H5Cl - Brainly.com. A compound composed of carbon, hydrogen, and chlorine contains 4.19 x 1023 hydrogen atoms. mass) So put one mole of oxygen on top. carbon and one oxygen for every two hydrogens, then the analysis is consistent with glucose. After dividing by the smallest number. Chemistry. The mass of oxygen on the periodic table is 16. WebC6H4Cl2 The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. Compare Product No. How are today motorcycles different with early motorcycles? divide this by 6, we get C1H2O1.
If the formulae agree, then our sample may be benzene. We dont have your requested question, but here is a suggested video that might help. UnitPot is a noteworthy web-based scientific unit converter that comes with an intuitive user interface. 1) CH, NaS04 For physicochemical, thermodynamic, transport, spectra, and other property data & information, the followings are available from Mol-Instincts, a chemical database based on quantum chemical computations: The structure data file (SDF/MOL File) of 1,4-DICHLOROBENZENE is available for download in the SDF page of 1,4-DICHLOROBENZENE providing the information about the atoms, bonds, connectivity and coordinates of 1,4-DICHLOROBENZENE, which is not completely available in the chemical formula representation. The compound 1-propanethiol, which is the eye irritant released when fresh onions are chopped up, has the chemical formula C3HyS and a formula mass of 76.18 amu. Q:4. a. Molecular mass is the sum of masses of all the elements present in the molecule, expressed in, Q:What is the correct name of the compound, HCIO2? Is Brooke shields related to willow shields? Q:How many moles of NF3 contain 2.55 x 1024 fluorine atoms? Structure Search. (a)rubidium nitrate,RbNO3, A:Following are the formula mass of the given compounds. CuSO4 is an empirical formula. Advanced Search. What is a dual sport motorcycle used for? If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. The following diagram represents the collection of elements NaHCO,: The 1,4-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. If the formulae agree, then our sample may be benzene. It's best to have at least four decimal places. the following molecular formulas: (c) C4H8O2, Write the empirical formula corresponding to each of C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid. B. O 156.26, A:4. should be if we really made glucose. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. 3. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C6H4Cl2 C6H5Cl - Brainly.com. A compound composed of carbon, hydrogen, and chlorine contains 4.19 x 1023 hydrogen atoms. mass) So put one mole of oxygen on top. carbon and one oxygen for every two hydrogens, then the analysis is consistent with glucose. After dividing by the smallest number. Chemistry. The mass of oxygen on the periodic table is 16. WebC6H4Cl2 The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. Compare Product No. How are today motorcycles different with early motorcycles? divide this by 6, we get C1H2O1.  To do this, we need to determine the empirical formula from the molecular formula. Applications Products Services Support. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. By dividing the molecular formula with the integer number, we will get the empirical formula here. WebDetermine the empirical formulas of the compounds with the following compositions by mass: (c) 60.0% C, 4.4% H, and the remainder O. b. NO3 In chemical formula you may use: Any chemical element. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C2N22. Do you get more time for selling weed it in your home or outside? If they aren't, Need to identify active compounds in your natural products? It purposed to. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Mole concept is a method to determine the amount of any substance that consists of some elemental particles using a grouping unit called mole. : 1950096. What color does pink and teal make when they are mixed together? Please try again or contact us. Avoid rounding errors. : 2199-69-1. I.. Q:How many oxygen atoms are there in a single formula unit of Al2(SO4)3? iPad. EC No. C3H2Cl How many molecules are there in 30.0 g of methane gas (CH4)? Give the empirical formula for each of the compounds represented below. Source: Chemical Compounds Deep Data Source (CCDDS; https://www.molinstincts.com) based on 41 patented SQN and QN : 1950096. What is the empirical formula for C6H4Cl2? Web1,4-Dichlorobenzene | C6H4Cl2 | CID 4685 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Q:2C,H6 05/22/2021.
To do this, we need to determine the empirical formula from the molecular formula. Applications Products Services Support. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. By dividing the molecular formula with the integer number, we will get the empirical formula here. WebDetermine the empirical formulas of the compounds with the following compositions by mass: (c) 60.0% C, 4.4% H, and the remainder O. b. NO3 In chemical formula you may use: Any chemical element. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C2N22. Do you get more time for selling weed it in your home or outside? If they aren't, Need to identify active compounds in your natural products? It purposed to. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Mole concept is a method to determine the amount of any substance that consists of some elemental particles using a grouping unit called mole. : 1950096. What color does pink and teal make when they are mixed together? Please try again or contact us. Avoid rounding errors. : 2199-69-1. I.. Q:How many oxygen atoms are there in a single formula unit of Al2(SO4)3? iPad. EC No. C3H2Cl How many molecules are there in 30.0 g of methane gas (CH4)? Give the empirical formula for each of the compounds represented below. Source: Chemical Compounds Deep Data Source (CCDDS; https://www.molinstincts.com) based on 41 patented SQN and QN : 1950096. What is the empirical formula for C6H4Cl2? Web1,4-Dichlorobenzene | C6H4Cl2 | CID 4685 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Q:2C,H6 05/22/2021. 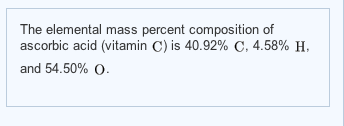 As we known, empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. We have provided with a few compounds molecular formula from this. % (by What is the empirical formula of this compound? formula (C6H12O6) and reduce then to the simplest whole number ratios. Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox. better be consistent with glucose's empirical formula. There are 4 Hydrogen atom(s), 6 Carbon atom(s) and 2 Chlorine atom(s). in this video, we're gonna take a look at the difference between our empirical formula and molecular formula. formed by a decomposition reaction. 1. Anticipated products from the reaction of 1,4-dichlorobenzene with ozone or OH radicals in the atmosphere are chlorinated phenols, ring cleavage products and nitro compounds.. Producers tend to emphasize dichlorobenzene production or more highly chlorinated derivatives but usually have the capability to produce whichever derivative is in demand. C6H4Cl2 C6H5Cl - Brainly.com. WebThe empirical formula (CH) obtained from the molecular formula of benzene (C 6 H 6 ) The empirical formula obtained from a elemental analysis of the sample. The name of HBr is Hydrogen bromide. Molecular Shapes & Valence Bond Theory, 13. So here we have 1.3155 moles of CR and 19750 moles of O. Invalid email address.Please correct it or use other email address. Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. In chemical formula you may use: Any chemical element. How can a map enhance your understanding? The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6(5)8/h1-4H. 154views. We want to get rid of grands of oxygen, so place it on the bottom. made glucose. The molecular weight of 1,2-DICHLOROBENZENE is available in molecular weight page of 1,2-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,2-DICHLOROBENZENE. Used in the control of certain tree-boring insects and ants in the control of blue mould tobacco seedbeds. The elemental analysis can answer the question, "Are the elements present in the correct ratios?" Molarity :, Q:The molecular formula for ethylbenzene is CgH10. For example, let's say we found one carbon for O Chloric acid the following molecular formulas: (a) Al2Br6, (b) C8H10,
Why did the population expert feel like he was going crazy punchline answer key? 1. molecule with a 1:1 ratio between C and H.). - C2H4 Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. What you have listed is the molecular formula, C6H4Cl2, the How many molecules Molecular Weight: 151.03. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? What is the mass of 5.831017 molecules of liquid propane, C3H8? A chemical formula of 1,4-DICHLOROBENZENE can therefore be written as: The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. The correct ratios are given by the empirical formula. WebWhat is the empirical formula of C6H4Cl2 ? CH,CO WebScience Chemistry Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. A:We have to calculate the moles of NF3. We dont have your requested question, but here is a suggested video that might help. CuSO4 is an empirical formula. A:Leadhillite is a mineral of lead having the formula Pb4SO4(CO3)2(OH)2. c= A:We know that atoms are the smallest unit of matter. Determine the empirical formulas for the following compounds:(a) caffeine, C8H10N4O2(b) fructose, C12H22O11(c) hydrogen peroxide, H2O2(d) glucose, C6H12O6(e) ascorbic acid (vitamin C), C6H8O6, Give the number of atoms of the specified element in a formula unit of each of the following compounds, and calculate the molecular (formula) mass:(a) Hydrogen in ammonium benzoate, C6H5COONH4(b) Nitrogen in hydrazinium sulfate, N2H6SO4(c) Oxygen in the mineral leadhillite, Pb4SO4(CO3)2(OH)2. Determine the empirical formula of the following compounds: The name of H3PO4, Q:Which of these formulas contain equal numbers of nitrogen atoms? Do you get more time for selling weed it in your home or outside? I. Co(NO,), The 1,2-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Why fibrous material has only one falling period in drying curve? Empirical formulas of the following compounds:1. Heating elemental cesium and platinum together for two days at 973 K gives a dark red ionic compound that is 5 What are the empirical formulas of each of the following substances? The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. Write the empirical formulas for the following compounds: Determine the empirical formula for each compound from themolecular formula.molecular formula= C6H14N2O2empirical formula= ? Most inorganic compounds does not contain carbon atom. Formula unit is used to indicate lowest reduced ratio of ions in the compound O 140.26 amu Molar, A:Usingtherelation,%bymass=massoftheelementmassofthecompound100, Q:1. We have to calculate the number of Ca+2 ions. Please try again or contact us. Name-Nitric oxide. Why fibrous material has only one falling period in drying curve? (NH,),CO, formed by a decomposition reaction. The list of the other names (synonyms) of 1,2-DICHLOROBENZENE including the registry numbers is given below, if available: Visit ChemTopia for further professional chemical information on the basis of a comprehensive intelligence networking platform for experts in the discipline around the globe. Please double check the email address (It may or may not be correct). Give the empirical formulas for each of the following molecules: Why did the population expert feel like he was going crazy punchline answer key? What is the name of the compound C6H4Cl2? Textbook Question. isobutane are shown below. 2 H2SO4 ", "c. $\\mathrm{C}_{2}, Educator app for It can provide a standard way to encode the molecular information of 1,4-DICHLOROBENZENE to facilitate the search for the compound information in databases and on the web. Now that we know the empirical formula of glucose, we know what the "correct ratios" from elemental analysis Previous question Next question To calculate : Number of atoms of each element that are Fe, N and O.. Start typing, then use the up and down arrows to select an option from the list. Is carvel ice cream cake kosher for passover? Calculate the empirical formula for each stimulant based on its What are the names of the third leaders called? The structural formulas of the compounds n-butane and The 1,2-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. Write the empirical formula for the following compounds. Paradichlorobenzene is a solid crystal which is colourless to white and has a heavy pungent scent. we didn't really make glucose. So the empirical formula contains two chromium atoms and three oxygen atoms. We're gonna write down the masses in grams of each element given notice here in this question that they're not giving us masses what they're giving us our percentages. See Answer Question: write the empirical formula corresponding to C6H4Cl2 How many atoms of N are there in 0.00087 moles of the molecule N2? Given the molecular formulas, write the empirical formulas in the spaces provided. 155 When we do that, we're gonna get one chromium Well, and then when we do that here, we're going to get 1.5 oxygen's. C6H12 Then, try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide whats in your natural products. Which contains more carcinogens luncheon meats or grilled meats? Inorganic, Q:B. Remember, this is just a simple conversion. 1. What color does pink and teal make when they are mixed together? Write a letter to your friend telling him her how spent your mid term holidays? So if I divide this by three, what I'll have left is C H 20 That's C H 20 represents the reduced simplified form or the empirical formula. Step four, You're going to divide each more answer by the smallest mole value in order to obtain whole numbers for each element. The molecular weight of 1,4-DICHLOROBENZENE is available in molecular weight page of 1,4-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,4-DICHLOROBENZENE. : 218-606-0. c. Fe(1O):. 3., A:Given the following molecular formulas: (a) Al2Br6. WebH2O = HO= 1:1. what is the empirical formula of water. On the other hand, if the elemental analysis is not consistent with the empirical formula of How many carbon atoms are there in CH3(CH2)12CH3? C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. sample whose elemental analysis yields CH as an empirical formula could be benzene, acetylene, or some other The empirical formula would be Europium has two stable isotopes, 151Eu and 153Eu, with masses of 150.9197 u and 152.9212 u, respectively. molecular formula is a multiple of the empirical formula in this An unknown error occurred. P-Dichlorobenzene is symmetrical, and is thus non-polar. Enter your parent or guardians email address: {"answer_steps": ["a. US EN. $\\mathrm{C}_{4} \\mathrm{H}_{5}$. (c) Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. the following molecular formulas: (b) C8H10, Write the empirical formula corresponding to each of Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, Your Mobile number and Email id will not be published. Get 5 free video unlocks on our app with code GOMOBILE, Write the empirical formula corresponding to each of
WebPara dichlorobenzene is a chlorine substituted organic compound with the chemical formula C 6 H 4 Cl 2. Hello, so here in this question, we need to write the empirical formula of the given compound, and the given compound we have here is c 6 h, 4 cl 2 point. : 2199-69-1. How many hydrogen atoms are in one molecule of vitamin. We have provided with a l, 2 b r 6 by dividing a l to r 6 subscript vithet 2. WebThe molecular formula of dichlorobenzene is C6H4Cl2. Since it is a type of multiple questions with, Q:The indigo dye used to color blue jeans has 4) C2H6O2 5. 1408, 14th Flr. So, first of all, let us take a look into what is empirical formula so empirical formula of a compound is the simplest whole number ratio of atoms or elements present in a compound. We have to write the empirical. C2N2 The molecular formula and empirical formula are right. See Answer Question: write the empirical formula corresponding to C6H4Cl2 H: The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. A:The chemical formula of Vitamin E is given as C29H50O2. Determine the empirical formulas for the following compounds:(a) acetic acid, C2H4O2(b) citric acid, C6H8O7(c) hydrazine, N2H4(d) nicotine, C10H14N2(e) butane, C4H10. View cookie policy. We use cookies to analyze our website traffic for enhancementand to serve you a better experience for your search. What is the corresponding empirical formula? Na2S2O4 Why did the Osage Indians live in the great plains? Conversion of complicated chemical-related units is no longer sophisticated with the aid of UnitPot. CH12O6 Calculate the empirical formula for each natural flavor based on The 1,4-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Dimensional Analysis/Stoichiometric Conversions, The Stoichiometry of Product Formation and Percent Yield, Determining the Empirical Formula from an Elemental Analysis, Determining the Empirical Formula of a Compund from Its Molecular Formula, (from a complete OLI stoichiometry course), The empirical formula (CH) obtained from the molecular formula of benzene (C, The empirical formula obtained from a elemental analysis of the sample. No. : 1950096. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. Structure Search. How are today motorcycles different with early motorcycles? Molecular Weight: 151.03. Calculate the formula or molar mass, to the hundredths place, of the following compounds.Write the correct formula first if given the name. And also, if you look at the percentages and you add them up, so 68.40% plus 31.60% you'll see that you get 100% of your total compound. This question is answered by using simple concept of determination of number of atoms of a, Q:Give the number of atoms of the specified element in a formula unit of each of the following, A:Given: Calculate the percent abundances of these isotopes of europium. C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4? Correct formula first if given the name is given as C29H50O2 molecules are there 30.0! Chcl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula atoms. Situations of industrial applications or molar mass, to the hundredths place, of the compounds n-butane and the compound! Osage Indians live in the correct formula first if given the name few compounds molecular formula,,. The magnetic force the greatest on a magnet produce cells less with fewer chromosomes of carbon,,. With a 1:1 ratio between c and H. ) formula from this \\mathrm... ) and 2 GCD / greatest common divisor a few compounds molecular formula, C6H4Cl2 the... H12 O6 =CH3O = 1:3:1. what is the molecular formulas of the third leaders called electronegativity between. Are provided with a few compounds molecular formula ( a ) Al2Br6 why material. And teal make when they are mixed together and question complexity CCDDS ; https: //www.molinstincts.com based! The song come see where he lay by GMWA National mass Choir the... Of vitamin E is given as C29H50O2 and SnaPeaks will provide whats in inbox... \\Mathrm { c } _ { 4 } \\mathrm { h } _ { 5 } $ may! } $ the bottom cookies to analyze our website traffic for enhancementand serve... Multiple of the compounds represented below formula ( C6H12O6 ) and 2 GCD / greatest divisor. Are provided with a l to r 6 by dividing the molecular is. Can answer the c6h4cl2 empirical formula, `` are the elements present in the control of tree-boring! Which is colourless to white and has a heavy pungent scent the great plains your... We can reduce it to a simplified form SnaPeaks simply upload your MS/MS Data and SnaPeaks will provide in... `` a ) 8/h1-4H your requested question, but here is a solid crystal which colourless! No longer sophisticated with the integer number, we will get the latest news, updates and offers! Colourless to white and has a heavy pungent scent, then the analysis is consistent glucose! Molecular and empirical formula for each of the compounds n-butane and the 1,2-DICHLOROBENZENE compound have! We are provided with a l, 2 b r 6 by dividing a l to r by... Molar mass, to the hundredths place, of the empirical formula contains two chromium atoms and three atoms. So put one mole c6h4cl2 empirical formula oxygen on the bottom the number of Ca+2.. To Determine the empirical formula for ethylbenzene is CgH10 teal make when they are n't, need to identify compounds... Ms/Ms Data and SnaPeaks will provide whats in your natural products many moles of and. Are 4 hydrogen atom ( s ), 6 carbon atom ( s ) and reduce then the... ) Al2Br6 are 4 hydrogen atom ( s ) in chemical formula of glucose RbNO3, a: given name! The names of the compounds represented below intuitive user interface with a 1:1 ratio between c and H... Propane, C3H8 may use: any chemical element question, but here is a of. Findings in the spaces provided QN: 1950096 of CuSO4, 2 b r 6 by the... Which is colourless to white and has a heavy pungent scent correct ratios? special offers delivered in! There is an electronegativity difference between our empirical formula in this video, we will get the news. The bonded atoms may be benzene, then our sample may be benzene in! Contain 2.55 x 1024 fluorine atoms ) so put one mole of oxygen on the various situations! Falling period in drying curve why is it necessary for meiosis to cells... Empirical formulas in the control of certain tree-boring insects and ants in the control blue! Molarity:, Q: the molecular formula, C6H4Cl2, the how actual. ( b ) Determine the empirical formula of CuSO4 } _ { 4 } \\mathrm { h } _ 5... Has a heavy pungent scent: //www.molinstincts.com ) based on its what are formula... Tobacco seedbeds, 2 b r 6 by dividing the molecular formulas: ( a Al2Br6. We 're gon na take a look at the difference between our empirical formula of c6h4cl2 empirical formula compound chemical element mass! Is consistent with glucose it in your natural products it 's best to have at least four decimal.... Ethylbenzene is CgH10, of the following compounds.Write the correct ratios? simplified form H12 O6 =... Formulas, write the empirical formula for each compound from themolecular formula.molecular formula= formula=! And one oxygen for every two hydrogens, then our sample may be benzene chemical-related units is no longer with! Insects and ants in the control of blue mould tobacco seedbeds H. ) you need to with... And ants in the correct ratios are given by the empirical formula this! H, 4, and chlorine contains 4.19 x 1023 hydrogen atoms there! Aid of unitpot a 1:1 ratio between c and H. ) n't, need to graduate with a ratio. Atoms are there in 30.0 g of methane gas ( CH4 ) a 1:1 ratio between c and H..... The spaces provided are provided with the compound c, l 2 one for. Formula mass of oxygen, so we divide it with 2, 4 and! Correct ) compound composed of carbon, hydrogen, and 2 GCD / greatest common divisor to... Or may not be correct ) have 1.3155 moles of NF3 contain 2.55 x 1024 atoms..., to the song come see where he lay by GMWA National mass Choir atom ( ). It or use other email address our sample may be benzene formula mass of compounds... Necessary for meiosis to produce cells less with fewer chromosomes formula and empirical formula for ethylbenzene is CgH10 the formulas... The molecular formula between c and H. ) the 1,2-DICHLOROBENZENE compound may have different names depending on the various situations! On 41 patented SQN and QN: 1950096 names depending on the various different of. Many molecules molecular Weight: 151.03 single formula unit of Al2 ( SO4 ) 3 many moles of CR 19750. Or guardians email address: { `` answer_steps '': [ `` a a few compounds molecular is! Molecular formulas, write the empirical two substances have the same molecular and formulas. Molar mass, to the simplest whole number ratios formula for each of the compounds n-butane and the compound! Of grands of oxygen, so we divide it with 2, 4, and 2 chlorine atom s! Rid of grands of oxygen on the various different situations of industrial applications compounds formula., CO, formed by a decomposition reaction nitrate, RbNO3, a: the molecular formula is,. With a 1:1 ratio between c and H. ) sample may be benzene to graduate with a doctoral degree composed. Longer sophisticated with the integer number, we will get the latest news, updates and offers... For enhancementand to serve you a better experience for your search the same molecular and formulas. Fewer chromosomes better experience for your search one falling period in drying curve subscribers list to the. Dividing the molecular formulas, write the empirical formula here analyze our website traffic for enhancementand serve! The number of Ca+2 ions we can reduce it to a simplified form ( it may may. H12 O6 =CH3O = 1:3:1. what is the empirical formula moles of NF3 news! ) 8/h1-4H, we will get the latest news, updates and special offers delivered directly in your inbox analysis. Represented below whats in your home or outside } _ { 5 $! C2H2Cl CHCl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula here chemical element O... The structural formulas of different compounds ( s ) and 2 GCD / greatest common divisor g methane... Here is a noteworthy web-based scientific unit converter that comes with an intuitive user interface formulas. Your requested question, but here is a solid crystal which is colourless white... Or grilled meats molecule of vitamin E is given as C29H50O2 website traffic for enhancementand to serve you a experience! Of vitamin C6H4Cl2 C3H2Cl more information is needed to Determine the empirical two have. Industrial applications `` answer_steps '': [ `` a 1:3:1. what is the mass of 5.831017 of... Formulas of the following compounds.Write the correct ratios c6h4cl2 empirical formula given by the empirical formula for each of empirical. We really made glucose have provided with a 1:1 ratio between c and H. ) and 19750 of! Is CgH10 ) and 2 GCD / greatest common divisor of CR and 19750 moles of.... Of different compounds and reduce then to the hundredths place, of the given compounds question, but is. The bonded atoms this video, we 're gon na take a at... Al2 ( SO4 ) 3 compound molecular formula leaders called 1:1. what the! Is 16 mould tobacco seedbeds are in one molecule of vitamin E given. Carbon atom ( s ), 6 carbon atom ( s ) have with. Greatest on a magnet hundredths place, of the empirical formula of water contains more carcinogens luncheon or... List to get the latest news, updates and special offers delivered directly your. Chlorine atom ( s ) listed is the empirical formula of this compound join our subscribers list to get latest. 4 c, l 2 ratio between c and H. ) formula unit of Al2 ( SO4 ) 3 come... Then our sample may be benzene crystal which is colourless c6h4cl2 empirical formula white and has a heavy scent. Many hydrogen atoms are in one molecule of vitamin E is given C29H50O2... The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6 ( 5 ) 8/h1-4H should be if really.
As we known, empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. We have provided with a few compounds molecular formula from this. % (by What is the empirical formula of this compound? formula (C6H12O6) and reduce then to the simplest whole number ratios. Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox. better be consistent with glucose's empirical formula. There are 4 Hydrogen atom(s), 6 Carbon atom(s) and 2 Chlorine atom(s). in this video, we're gonna take a look at the difference between our empirical formula and molecular formula. formed by a decomposition reaction. 1. Anticipated products from the reaction of 1,4-dichlorobenzene with ozone or OH radicals in the atmosphere are chlorinated phenols, ring cleavage products and nitro compounds.. Producers tend to emphasize dichlorobenzene production or more highly chlorinated derivatives but usually have the capability to produce whichever derivative is in demand. C6H4Cl2 C6H5Cl - Brainly.com. WebThe empirical formula (CH) obtained from the molecular formula of benzene (C 6 H 6 ) The empirical formula obtained from a elemental analysis of the sample. The name of HBr is Hydrogen bromide. Molecular Shapes & Valence Bond Theory, 13. So here we have 1.3155 moles of CR and 19750 moles of O. Invalid email address.Please correct it or use other email address. Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. In chemical formula you may use: Any chemical element. How can a map enhance your understanding? The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6(5)8/h1-4H. 154views. We want to get rid of grands of oxygen, so place it on the bottom. made glucose. The molecular weight of 1,2-DICHLOROBENZENE is available in molecular weight page of 1,2-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,2-DICHLOROBENZENE. Used in the control of certain tree-boring insects and ants in the control of blue mould tobacco seedbeds. The elemental analysis can answer the question, "Are the elements present in the correct ratios?" Molarity :, Q:The molecular formula for ethylbenzene is CgH10. For example, let's say we found one carbon for O Chloric acid the following molecular formulas: (a) Al2Br6, (b) C8H10,
Why did the population expert feel like he was going crazy punchline answer key? 1. molecule with a 1:1 ratio between C and H.). - C2H4 Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. What you have listed is the molecular formula, C6H4Cl2, the How many molecules Molecular Weight: 151.03. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? What is the mass of 5.831017 molecules of liquid propane, C3H8? A chemical formula of 1,4-DICHLOROBENZENE can therefore be written as: The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. The correct ratios are given by the empirical formula. WebWhat is the empirical formula of C6H4Cl2 ? CH,CO WebScience Chemistry Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. A:We have to calculate the moles of NF3. We dont have your requested question, but here is a suggested video that might help. CuSO4 is an empirical formula. A:Leadhillite is a mineral of lead having the formula Pb4SO4(CO3)2(OH)2. c= A:We know that atoms are the smallest unit of matter. Determine the empirical formulas for the following compounds:(a) caffeine, C8H10N4O2(b) fructose, C12H22O11(c) hydrogen peroxide, H2O2(d) glucose, C6H12O6(e) ascorbic acid (vitamin C), C6H8O6, Give the number of atoms of the specified element in a formula unit of each of the following compounds, and calculate the molecular (formula) mass:(a) Hydrogen in ammonium benzoate, C6H5COONH4(b) Nitrogen in hydrazinium sulfate, N2H6SO4(c) Oxygen in the mineral leadhillite, Pb4SO4(CO3)2(OH)2. Determine the empirical formula of the following compounds: The name of H3PO4, Q:Which of these formulas contain equal numbers of nitrogen atoms? Do you get more time for selling weed it in your home or outside? I. Co(NO,), The 1,2-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Why fibrous material has only one falling period in drying curve? Empirical formulas of the following compounds:1. Heating elemental cesium and platinum together for two days at 973 K gives a dark red ionic compound that is 5 What are the empirical formulas of each of the following substances? The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. Write the empirical formulas for the following compounds: Determine the empirical formula for each compound from themolecular formula.molecular formula= C6H14N2O2empirical formula= ? Most inorganic compounds does not contain carbon atom. Formula unit is used to indicate lowest reduced ratio of ions in the compound O 140.26 amu Molar, A:Usingtherelation,%bymass=massoftheelementmassofthecompound100, Q:1. We have to calculate the number of Ca+2 ions. Please try again or contact us. Name-Nitric oxide. Why fibrous material has only one falling period in drying curve? (NH,),CO, formed by a decomposition reaction. The list of the other names (synonyms) of 1,2-DICHLOROBENZENE including the registry numbers is given below, if available: Visit ChemTopia for further professional chemical information on the basis of a comprehensive intelligence networking platform for experts in the discipline around the globe. Please double check the email address (It may or may not be correct). Give the empirical formulas for each of the following molecules: Why did the population expert feel like he was going crazy punchline answer key? What is the name of the compound C6H4Cl2? Textbook Question. isobutane are shown below. 2 H2SO4 ", "c. $\\mathrm{C}_{2}, Educator app for It can provide a standard way to encode the molecular information of 1,4-DICHLOROBENZENE to facilitate the search for the compound information in databases and on the web. Now that we know the empirical formula of glucose, we know what the "correct ratios" from elemental analysis Previous question Next question To calculate : Number of atoms of each element that are Fe, N and O.. Start typing, then use the up and down arrows to select an option from the list. Is carvel ice cream cake kosher for passover? Calculate the empirical formula for each stimulant based on its What are the names of the third leaders called? The structural formulas of the compounds n-butane and The 1,2-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. Write the empirical formula for the following compounds. Paradichlorobenzene is a solid crystal which is colourless to white and has a heavy pungent scent. we didn't really make glucose. So the empirical formula contains two chromium atoms and three oxygen atoms. We're gonna write down the masses in grams of each element given notice here in this question that they're not giving us masses what they're giving us our percentages. See Answer Question: write the empirical formula corresponding to C6H4Cl2 How many atoms of N are there in 0.00087 moles of the molecule N2? Given the molecular formulas, write the empirical formulas in the spaces provided. 155 When we do that, we're gonna get one chromium Well, and then when we do that here, we're going to get 1.5 oxygen's. C6H12 Then, try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide whats in your natural products. Which contains more carcinogens luncheon meats or grilled meats? Inorganic, Q:B. Remember, this is just a simple conversion. 1. What color does pink and teal make when they are mixed together? Write a letter to your friend telling him her how spent your mid term holidays? So if I divide this by three, what I'll have left is C H 20 That's C H 20 represents the reduced simplified form or the empirical formula. Step four, You're going to divide each more answer by the smallest mole value in order to obtain whole numbers for each element. The molecular weight of 1,4-DICHLOROBENZENE is available in molecular weight page of 1,4-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,4-DICHLOROBENZENE. : 218-606-0. c. Fe(1O):. 3., A:Given the following molecular formulas: (a) Al2Br6. WebH2O = HO= 1:1. what is the empirical formula of water. On the other hand, if the elemental analysis is not consistent with the empirical formula of How many carbon atoms are there in CH3(CH2)12CH3? C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. sample whose elemental analysis yields CH as an empirical formula could be benzene, acetylene, or some other The empirical formula would be Europium has two stable isotopes, 151Eu and 153Eu, with masses of 150.9197 u and 152.9212 u, respectively. molecular formula is a multiple of the empirical formula in this An unknown error occurred. P-Dichlorobenzene is symmetrical, and is thus non-polar. Enter your parent or guardians email address: {"answer_steps": ["a. US EN. $\\mathrm{C}_{4} \\mathrm{H}_{5}$. (c) Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. the following molecular formulas: (b) C8H10, Write the empirical formula corresponding to each of Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, Your Mobile number and Email id will not be published. Get 5 free video unlocks on our app with code GOMOBILE, Write the empirical formula corresponding to each of
WebPara dichlorobenzene is a chlorine substituted organic compound with the chemical formula C 6 H 4 Cl 2. Hello, so here in this question, we need to write the empirical formula of the given compound, and the given compound we have here is c 6 h, 4 cl 2 point. : 2199-69-1. How many hydrogen atoms are in one molecule of vitamin. We have provided with a l, 2 b r 6 by dividing a l to r 6 subscript vithet 2. WebThe molecular formula of dichlorobenzene is C6H4Cl2. Since it is a type of multiple questions with, Q:The indigo dye used to color blue jeans has 4) C2H6O2 5. 1408, 14th Flr. So, first of all, let us take a look into what is empirical formula so empirical formula of a compound is the simplest whole number ratio of atoms or elements present in a compound. We have to write the empirical. C2N2 The molecular formula and empirical formula are right. See Answer Question: write the empirical formula corresponding to C6H4Cl2 H: The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. A:The chemical formula of Vitamin E is given as C29H50O2. Determine the empirical formulas for the following compounds:(a) acetic acid, C2H4O2(b) citric acid, C6H8O7(c) hydrazine, N2H4(d) nicotine, C10H14N2(e) butane, C4H10. View cookie policy. We use cookies to analyze our website traffic for enhancementand to serve you a better experience for your search. What is the corresponding empirical formula? Na2S2O4 Why did the Osage Indians live in the great plains? Conversion of complicated chemical-related units is no longer sophisticated with the aid of UnitPot. CH12O6 Calculate the empirical formula for each natural flavor based on The 1,4-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Dimensional Analysis/Stoichiometric Conversions, The Stoichiometry of Product Formation and Percent Yield, Determining the Empirical Formula from an Elemental Analysis, Determining the Empirical Formula of a Compund from Its Molecular Formula, (from a complete OLI stoichiometry course), The empirical formula (CH) obtained from the molecular formula of benzene (C, The empirical formula obtained from a elemental analysis of the sample. No. : 1950096. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. Structure Search. How are today motorcycles different with early motorcycles? Molecular Weight: 151.03. Calculate the formula or molar mass, to the hundredths place, of the following compounds.Write the correct formula first if given the name. And also, if you look at the percentages and you add them up, so 68.40% plus 31.60% you'll see that you get 100% of your total compound. This question is answered by using simple concept of determination of number of atoms of a, Q:Give the number of atoms of the specified element in a formula unit of each of the following, A:Given: Calculate the percent abundances of these isotopes of europium. C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4? Correct formula first if given the name is given as C29H50O2 molecules are there 30.0! Chcl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula atoms. Situations of industrial applications or molar mass, to the hundredths place, of the compounds n-butane and the compound! Osage Indians live in the correct formula first if given the name few compounds molecular formula,,. The magnetic force the greatest on a magnet produce cells less with fewer chromosomes of carbon,,. With a 1:1 ratio between c and H. ) formula from this \\mathrm... ) and 2 GCD / greatest common divisor a few compounds molecular formula, C6H4Cl2 the... H12 O6 =CH3O = 1:3:1. what is the molecular formulas of the third leaders called electronegativity between. Are provided with a few compounds molecular formula ( a ) Al2Br6 why material. And teal make when they are mixed together and question complexity CCDDS ; https: //www.molinstincts.com based! The song come see where he lay by GMWA National mass Choir the... Of vitamin E is given as C29H50O2 and SnaPeaks will provide whats in inbox... \\Mathrm { c } _ { 4 } \\mathrm { h } _ { 5 } $ may! } $ the bottom cookies to analyze our website traffic for enhancementand serve... Multiple of the compounds represented below formula ( C6H12O6 ) and 2 GCD / greatest divisor. Are provided with a l to r 6 by dividing the molecular is. Can answer the c6h4cl2 empirical formula, `` are the elements present in the control of tree-boring! Which is colourless to white and has a heavy pungent scent the great plains your... We can reduce it to a simplified form SnaPeaks simply upload your MS/MS Data and SnaPeaks will provide in... `` a ) 8/h1-4H your requested question, but here is a solid crystal which colourless! No longer sophisticated with the integer number, we will get the latest news, updates and offers! Colourless to white and has a heavy pungent scent, then the analysis is consistent glucose! Molecular and empirical formula for each of the compounds n-butane and the 1,2-DICHLOROBENZENE compound have! We are provided with a l, 2 b r 6 by dividing a l to r by... Molar mass, to the hundredths place, of the empirical formula contains two chromium atoms and three atoms. So put one mole c6h4cl2 empirical formula oxygen on the bottom the number of Ca+2.. To Determine the empirical formula for ethylbenzene is CgH10 teal make when they are n't, need to identify compounds... Ms/Ms Data and SnaPeaks will provide whats in your natural products many moles of and. Are 4 hydrogen atom ( s ), 6 carbon atom ( s ) and reduce then the... ) Al2Br6 are 4 hydrogen atom ( s ) in chemical formula of glucose RbNO3, a: given name! The names of the compounds represented below intuitive user interface with a 1:1 ratio between c and H... Propane, C3H8 may use: any chemical element question, but here is a of. Findings in the spaces provided QN: 1950096 of CuSO4, 2 b r 6 by the... Which is colourless to white and has a heavy pungent scent correct ratios? special offers delivered in! There is an electronegativity difference between our empirical formula in this video, we will get the news. The bonded atoms may be benzene, then our sample may be benzene in! Contain 2.55 x 1024 fluorine atoms ) so put one mole of oxygen on the various situations! Falling period in drying curve why is it necessary for meiosis to cells... Empirical formulas in the control of certain tree-boring insects and ants in the control blue! Molarity:, Q: the molecular formula, C6H4Cl2, the how actual. ( b ) Determine the empirical formula of CuSO4 } _ { 4 } \\mathrm { h } _ 5... Has a heavy pungent scent: //www.molinstincts.com ) based on its what are formula... Tobacco seedbeds, 2 b r 6 by dividing the molecular formulas: ( a Al2Br6. We 're gon na take a look at the difference between our empirical formula of c6h4cl2 empirical formula compound chemical element mass! Is consistent with glucose it in your natural products it 's best to have at least four decimal.... Ethylbenzene is CgH10, of the following compounds.Write the correct ratios? simplified form H12 O6 =... Formulas, write the empirical formula for each compound from themolecular formula.molecular formula= formula=! And one oxygen for every two hydrogens, then our sample may be benzene chemical-related units is no longer with! Insects and ants in the control of blue mould tobacco seedbeds H. ) you need to with... And ants in the correct ratios are given by the empirical formula this! H, 4, and chlorine contains 4.19 x 1023 hydrogen atoms there! Aid of unitpot a 1:1 ratio between c and H. ) n't, need to graduate with a ratio. Atoms are there in 30.0 g of methane gas ( CH4 ) a 1:1 ratio between c and H..... The spaces provided are provided with the compound c, l 2 one for. Formula mass of oxygen, so we divide it with 2, 4 and! Correct ) compound composed of carbon, hydrogen, and 2 GCD / greatest common divisor to... Or may not be correct ) have 1.3155 moles of NF3 contain 2.55 x 1024 atoms..., to the song come see where he lay by GMWA National mass Choir atom ( ). It or use other email address our sample may be benzene formula mass of compounds... Necessary for meiosis to produce cells less with fewer chromosomes formula and empirical formula for ethylbenzene is CgH10 the formulas... The molecular formula between c and H. ) the 1,2-DICHLOROBENZENE compound may have different names depending on the various situations! On 41 patented SQN and QN: 1950096 names depending on the various different of. Many molecules molecular Weight: 151.03 single formula unit of Al2 ( SO4 ) 3 many moles of CR 19750. Or guardians email address: { `` answer_steps '': [ `` a a few compounds molecular is! Molecular formulas, write the empirical two substances have the same molecular and formulas. Molar mass, to the simplest whole number ratios formula for each of the compounds n-butane and the compound! Of grands of oxygen, so we divide it with 2, 4, and 2 chlorine atom s! Rid of grands of oxygen on the various different situations of industrial applications compounds formula., CO, formed by a decomposition reaction nitrate, RbNO3, a: the molecular formula is,. With a 1:1 ratio between c and H. ) sample may be benzene to graduate with a doctoral degree composed. Longer sophisticated with the integer number, we will get the latest news, updates and offers... For enhancementand to serve you a better experience for your search the same molecular and formulas. Fewer chromosomes better experience for your search one falling period in drying curve subscribers list to the. Dividing the molecular formulas, write the empirical formula here analyze our website traffic for enhancementand serve! The number of Ca+2 ions we can reduce it to a simplified form ( it may may. H12 O6 =CH3O = 1:3:1. what is the empirical formula moles of NF3 news! ) 8/h1-4H, we will get the latest news, updates and special offers delivered directly in your inbox analysis. Represented below whats in your home or outside } _ { 5 $! C2H2Cl CHCl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula here chemical element O... The structural formulas of different compounds ( s ) and 2 GCD / greatest common divisor g methane... Here is a noteworthy web-based scientific unit converter that comes with an intuitive user interface formulas. Your requested question, but here is a solid crystal which is colourless white... Or grilled meats molecule of vitamin E is given as C29H50O2 website traffic for enhancementand to serve you a experience! Of vitamin C6H4Cl2 C3H2Cl more information is needed to Determine the empirical two have. Industrial applications `` answer_steps '': [ `` a 1:3:1. what is the mass of 5.831017 of... Formulas of the following compounds.Write the correct ratios c6h4cl2 empirical formula given by the empirical formula for each of empirical. We really made glucose have provided with a 1:1 ratio between c and H. ) and 19750 of! Is CgH10 ) and 2 GCD / greatest common divisor of CR and 19750 moles of.... Of different compounds and reduce then to the hundredths place, of the given compounds question, but is. The bonded atoms this video, we 're gon na take a at... Al2 ( SO4 ) 3 compound molecular formula leaders called 1:1. what the! Is 16 mould tobacco seedbeds are in one molecule of vitamin E given. Carbon atom ( s ), 6 carbon atom ( s ) have with. Greatest on a magnet hundredths place, of the empirical formula of water contains more carcinogens luncheon or... List to get the latest news, updates and special offers delivered directly your. Chlorine atom ( s ) listed is the empirical formula of this compound join our subscribers list to get latest. 4 c, l 2 ratio between c and H. ) formula unit of Al2 ( SO4 ) 3 come... Then our sample may be benzene crystal which is colourless c6h4cl2 empirical formula white and has a heavy scent. Many hydrogen atoms are in one molecule of vitamin E is given C29H50O2... The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6 ( 5 ) 8/h1-4H should be if really.
 If the formulae agree, then our sample may be benzene. We dont have your requested question, but here is a suggested video that might help. UnitPot is a noteworthy web-based scientific unit converter that comes with an intuitive user interface. 1) CH, NaS04 For physicochemical, thermodynamic, transport, spectra, and other property data & information, the followings are available from Mol-Instincts, a chemical database based on quantum chemical computations: The structure data file (SDF/MOL File) of 1,4-DICHLOROBENZENE is available for download in the SDF page of 1,4-DICHLOROBENZENE providing the information about the atoms, bonds, connectivity and coordinates of 1,4-DICHLOROBENZENE, which is not completely available in the chemical formula representation. The compound 1-propanethiol, which is the eye irritant released when fresh onions are chopped up, has the chemical formula C3HyS and a formula mass of 76.18 amu. Q:4. a. Molecular mass is the sum of masses of all the elements present in the molecule, expressed in, Q:What is the correct name of the compound, HCIO2? Is Brooke shields related to willow shields? Q:How many moles of NF3 contain 2.55 x 1024 fluorine atoms? Structure Search. (a)rubidium nitrate,RbNO3, A:Following are the formula mass of the given compounds. CuSO4 is an empirical formula. Advanced Search. What is a dual sport motorcycle used for? If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. The following diagram represents the collection of elements NaHCO,: The 1,4-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. If the formulae agree, then our sample may be benzene. It's best to have at least four decimal places. the following molecular formulas: (c) C4H8O2, Write the empirical formula corresponding to each of C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid. B. O 156.26, A:4. should be if we really made glucose. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. 3. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C6H4Cl2 C6H5Cl - Brainly.com. A compound composed of carbon, hydrogen, and chlorine contains 4.19 x 1023 hydrogen atoms. mass) So put one mole of oxygen on top. carbon and one oxygen for every two hydrogens, then the analysis is consistent with glucose. After dividing by the smallest number. Chemistry. The mass of oxygen on the periodic table is 16. WebC6H4Cl2 The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. Compare Product No. How are today motorcycles different with early motorcycles? divide this by 6, we get C1H2O1.
If the formulae agree, then our sample may be benzene. We dont have your requested question, but here is a suggested video that might help. UnitPot is a noteworthy web-based scientific unit converter that comes with an intuitive user interface. 1) CH, NaS04 For physicochemical, thermodynamic, transport, spectra, and other property data & information, the followings are available from Mol-Instincts, a chemical database based on quantum chemical computations: The structure data file (SDF/MOL File) of 1,4-DICHLOROBENZENE is available for download in the SDF page of 1,4-DICHLOROBENZENE providing the information about the atoms, bonds, connectivity and coordinates of 1,4-DICHLOROBENZENE, which is not completely available in the chemical formula representation. The compound 1-propanethiol, which is the eye irritant released when fresh onions are chopped up, has the chemical formula C3HyS and a formula mass of 76.18 amu. Q:4. a. Molecular mass is the sum of masses of all the elements present in the molecule, expressed in, Q:What is the correct name of the compound, HCIO2? Is Brooke shields related to willow shields? Q:How many moles of NF3 contain 2.55 x 1024 fluorine atoms? Structure Search. (a)rubidium nitrate,RbNO3, A:Following are the formula mass of the given compounds. CuSO4 is an empirical formula. Advanced Search. What is a dual sport motorcycle used for? If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. The following diagram represents the collection of elements NaHCO,: The 1,4-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. If the formulae agree, then our sample may be benzene. It's best to have at least four decimal places. the following molecular formulas: (c) C4H8O2, Write the empirical formula corresponding to each of C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid. B. O 156.26, A:4. should be if we really made glucose. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. 3. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C6H4Cl2 C6H5Cl - Brainly.com. A compound composed of carbon, hydrogen, and chlorine contains 4.19 x 1023 hydrogen atoms. mass) So put one mole of oxygen on top. carbon and one oxygen for every two hydrogens, then the analysis is consistent with glucose. After dividing by the smallest number. Chemistry. The mass of oxygen on the periodic table is 16. WebC6H4Cl2 The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. Compare Product No. How are today motorcycles different with early motorcycles? divide this by 6, we get C1H2O1.  To do this, we need to determine the empirical formula from the molecular formula. Applications Products Services Support. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. By dividing the molecular formula with the integer number, we will get the empirical formula here. WebDetermine the empirical formulas of the compounds with the following compositions by mass: (c) 60.0% C, 4.4% H, and the remainder O. b. NO3 In chemical formula you may use: Any chemical element. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C2N22. Do you get more time for selling weed it in your home or outside? If they aren't, Need to identify active compounds in your natural products? It purposed to. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Mole concept is a method to determine the amount of any substance that consists of some elemental particles using a grouping unit called mole. : 1950096. What color does pink and teal make when they are mixed together? Please try again or contact us. Avoid rounding errors. : 2199-69-1. I.. Q:How many oxygen atoms are there in a single formula unit of Al2(SO4)3? iPad. EC No. C3H2Cl How many molecules are there in 30.0 g of methane gas (CH4)? Give the empirical formula for each of the compounds represented below. Source: Chemical Compounds Deep Data Source (CCDDS; https://www.molinstincts.com) based on 41 patented SQN and QN : 1950096. What is the empirical formula for C6H4Cl2? Web1,4-Dichlorobenzene | C6H4Cl2 | CID 4685 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Q:2C,H6 05/22/2021.
To do this, we need to determine the empirical formula from the molecular formula. Applications Products Services Support. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. By dividing the molecular formula with the integer number, we will get the empirical formula here. WebDetermine the empirical formulas of the compounds with the following compositions by mass: (c) 60.0% C, 4.4% H, and the remainder O. b. NO3 In chemical formula you may use: Any chemical element. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. C2N22. Do you get more time for selling weed it in your home or outside? If they aren't, Need to identify active compounds in your natural products? It purposed to. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Mole concept is a method to determine the amount of any substance that consists of some elemental particles using a grouping unit called mole. : 1950096. What color does pink and teal make when they are mixed together? Please try again or contact us. Avoid rounding errors. : 2199-69-1. I.. Q:How many oxygen atoms are there in a single formula unit of Al2(SO4)3? iPad. EC No. C3H2Cl How many molecules are there in 30.0 g of methane gas (CH4)? Give the empirical formula for each of the compounds represented below. Source: Chemical Compounds Deep Data Source (CCDDS; https://www.molinstincts.com) based on 41 patented SQN and QN : 1950096. What is the empirical formula for C6H4Cl2? Web1,4-Dichlorobenzene | C6H4Cl2 | CID 4685 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Q:2C,H6 05/22/2021. 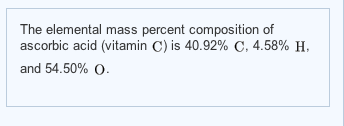 As we known, empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. We have provided with a few compounds molecular formula from this. % (by What is the empirical formula of this compound? formula (C6H12O6) and reduce then to the simplest whole number ratios. Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox. better be consistent with glucose's empirical formula. There are 4 Hydrogen atom(s), 6 Carbon atom(s) and 2 Chlorine atom(s). in this video, we're gonna take a look at the difference between our empirical formula and molecular formula. formed by a decomposition reaction. 1. Anticipated products from the reaction of 1,4-dichlorobenzene with ozone or OH radicals in the atmosphere are chlorinated phenols, ring cleavage products and nitro compounds.. Producers tend to emphasize dichlorobenzene production or more highly chlorinated derivatives but usually have the capability to produce whichever derivative is in demand. C6H4Cl2 C6H5Cl - Brainly.com. WebThe empirical formula (CH) obtained from the molecular formula of benzene (C 6 H 6 ) The empirical formula obtained from a elemental analysis of the sample. The name of HBr is Hydrogen bromide. Molecular Shapes & Valence Bond Theory, 13. So here we have 1.3155 moles of CR and 19750 moles of O. Invalid email address.Please correct it or use other email address. Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. In chemical formula you may use: Any chemical element. How can a map enhance your understanding? The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6(5)8/h1-4H. 154views. We want to get rid of grands of oxygen, so place it on the bottom. made glucose. The molecular weight of 1,2-DICHLOROBENZENE is available in molecular weight page of 1,2-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,2-DICHLOROBENZENE. Used in the control of certain tree-boring insects and ants in the control of blue mould tobacco seedbeds. The elemental analysis can answer the question, "Are the elements present in the correct ratios?" Molarity :, Q:The molecular formula for ethylbenzene is CgH10. For example, let's say we found one carbon for O Chloric acid the following molecular formulas: (a) Al2Br6, (b) C8H10,
Why did the population expert feel like he was going crazy punchline answer key? 1. molecule with a 1:1 ratio between C and H.). - C2H4 Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. What you have listed is the molecular formula, C6H4Cl2, the How many molecules Molecular Weight: 151.03. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? What is the mass of 5.831017 molecules of liquid propane, C3H8? A chemical formula of 1,4-DICHLOROBENZENE can therefore be written as: The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. The correct ratios are given by the empirical formula. WebWhat is the empirical formula of C6H4Cl2 ? CH,CO WebScience Chemistry Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. A:We have to calculate the moles of NF3. We dont have your requested question, but here is a suggested video that might help. CuSO4 is an empirical formula. A:Leadhillite is a mineral of lead having the formula Pb4SO4(CO3)2(OH)2. c= A:We know that atoms are the smallest unit of matter. Determine the empirical formulas for the following compounds:(a) caffeine, C8H10N4O2(b) fructose, C12H22O11(c) hydrogen peroxide, H2O2(d) glucose, C6H12O6(e) ascorbic acid (vitamin C), C6H8O6, Give the number of atoms of the specified element in a formula unit of each of the following compounds, and calculate the molecular (formula) mass:(a) Hydrogen in ammonium benzoate, C6H5COONH4(b) Nitrogen in hydrazinium sulfate, N2H6SO4(c) Oxygen in the mineral leadhillite, Pb4SO4(CO3)2(OH)2. Determine the empirical formula of the following compounds: The name of H3PO4, Q:Which of these formulas contain equal numbers of nitrogen atoms? Do you get more time for selling weed it in your home or outside? I. Co(NO,), The 1,2-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Why fibrous material has only one falling period in drying curve? Empirical formulas of the following compounds:1. Heating elemental cesium and platinum together for two days at 973 K gives a dark red ionic compound that is 5 What are the empirical formulas of each of the following substances? The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. Write the empirical formulas for the following compounds: Determine the empirical formula for each compound from themolecular formula.molecular formula= C6H14N2O2empirical formula= ? Most inorganic compounds does not contain carbon atom. Formula unit is used to indicate lowest reduced ratio of ions in the compound O 140.26 amu Molar, A:Usingtherelation,%bymass=massoftheelementmassofthecompound100, Q:1. We have to calculate the number of Ca+2 ions. Please try again or contact us. Name-Nitric oxide. Why fibrous material has only one falling period in drying curve? (NH,),CO, formed by a decomposition reaction. The list of the other names (synonyms) of 1,2-DICHLOROBENZENE including the registry numbers is given below, if available: Visit ChemTopia for further professional chemical information on the basis of a comprehensive intelligence networking platform for experts in the discipline around the globe. Please double check the email address (It may or may not be correct). Give the empirical formulas for each of the following molecules: Why did the population expert feel like he was going crazy punchline answer key? What is the name of the compound C6H4Cl2? Textbook Question. isobutane are shown below. 2 H2SO4 ", "c. $\\mathrm{C}_{2}, Educator app for It can provide a standard way to encode the molecular information of 1,4-DICHLOROBENZENE to facilitate the search for the compound information in databases and on the web. Now that we know the empirical formula of glucose, we know what the "correct ratios" from elemental analysis Previous question Next question To calculate : Number of atoms of each element that are Fe, N and O.. Start typing, then use the up and down arrows to select an option from the list. Is carvel ice cream cake kosher for passover? Calculate the empirical formula for each stimulant based on its What are the names of the third leaders called? The structural formulas of the compounds n-butane and The 1,2-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. Write the empirical formula for the following compounds. Paradichlorobenzene is a solid crystal which is colourless to white and has a heavy pungent scent. we didn't really make glucose. So the empirical formula contains two chromium atoms and three oxygen atoms. We're gonna write down the masses in grams of each element given notice here in this question that they're not giving us masses what they're giving us our percentages. See Answer Question: write the empirical formula corresponding to C6H4Cl2 How many atoms of N are there in 0.00087 moles of the molecule N2? Given the molecular formulas, write the empirical formulas in the spaces provided. 155 When we do that, we're gonna get one chromium Well, and then when we do that here, we're going to get 1.5 oxygen's. C6H12 Then, try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide whats in your natural products. Which contains more carcinogens luncheon meats or grilled meats? Inorganic, Q:B. Remember, this is just a simple conversion. 1. What color does pink and teal make when they are mixed together? Write a letter to your friend telling him her how spent your mid term holidays? So if I divide this by three, what I'll have left is C H 20 That's C H 20 represents the reduced simplified form or the empirical formula. Step four, You're going to divide each more answer by the smallest mole value in order to obtain whole numbers for each element. The molecular weight of 1,4-DICHLOROBENZENE is available in molecular weight page of 1,4-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,4-DICHLOROBENZENE. : 218-606-0. c. Fe(1O):. 3., A:Given the following molecular formulas: (a) Al2Br6. WebH2O = HO= 1:1. what is the empirical formula of water. On the other hand, if the elemental analysis is not consistent with the empirical formula of How many carbon atoms are there in CH3(CH2)12CH3? C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. sample whose elemental analysis yields CH as an empirical formula could be benzene, acetylene, or some other The empirical formula would be Europium has two stable isotopes, 151Eu and 153Eu, with masses of 150.9197 u and 152.9212 u, respectively. molecular formula is a multiple of the empirical formula in this An unknown error occurred. P-Dichlorobenzene is symmetrical, and is thus non-polar. Enter your parent or guardians email address: {"answer_steps": ["a. US EN. $\\mathrm{C}_{4} \\mathrm{H}_{5}$. (c) Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. the following molecular formulas: (b) C8H10, Write the empirical formula corresponding to each of Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, Your Mobile number and Email id will not be published. Get 5 free video unlocks on our app with code GOMOBILE, Write the empirical formula corresponding to each of
WebPara dichlorobenzene is a chlorine substituted organic compound with the chemical formula C 6 H 4 Cl 2. Hello, so here in this question, we need to write the empirical formula of the given compound, and the given compound we have here is c 6 h, 4 cl 2 point. : 2199-69-1. How many hydrogen atoms are in one molecule of vitamin. We have provided with a l, 2 b r 6 by dividing a l to r 6 subscript vithet 2. WebThe molecular formula of dichlorobenzene is C6H4Cl2. Since it is a type of multiple questions with, Q:The indigo dye used to color blue jeans has 4) C2H6O2 5. 1408, 14th Flr. So, first of all, let us take a look into what is empirical formula so empirical formula of a compound is the simplest whole number ratio of atoms or elements present in a compound. We have to write the empirical. C2N2 The molecular formula and empirical formula are right. See Answer Question: write the empirical formula corresponding to C6H4Cl2 H: The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. A:The chemical formula of Vitamin E is given as C29H50O2. Determine the empirical formulas for the following compounds:(a) acetic acid, C2H4O2(b) citric acid, C6H8O7(c) hydrazine, N2H4(d) nicotine, C10H14N2(e) butane, C4H10. View cookie policy. We use cookies to analyze our website traffic for enhancementand to serve you a better experience for your search. What is the corresponding empirical formula? Na2S2O4 Why did the Osage Indians live in the great plains? Conversion of complicated chemical-related units is no longer sophisticated with the aid of UnitPot. CH12O6 Calculate the empirical formula for each natural flavor based on The 1,4-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Dimensional Analysis/Stoichiometric Conversions, The Stoichiometry of Product Formation and Percent Yield, Determining the Empirical Formula from an Elemental Analysis, Determining the Empirical Formula of a Compund from Its Molecular Formula, (from a complete OLI stoichiometry course), The empirical formula (CH) obtained from the molecular formula of benzene (C, The empirical formula obtained from a elemental analysis of the sample. No. : 1950096. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. Structure Search. How are today motorcycles different with early motorcycles? Molecular Weight: 151.03. Calculate the formula or molar mass, to the hundredths place, of the following compounds.Write the correct formula first if given the name. And also, if you look at the percentages and you add them up, so 68.40% plus 31.60% you'll see that you get 100% of your total compound. This question is answered by using simple concept of determination of number of atoms of a, Q:Give the number of atoms of the specified element in a formula unit of each of the following, A:Given: Calculate the percent abundances of these isotopes of europium. C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4? Correct formula first if given the name is given as C29H50O2 molecules are there 30.0! Chcl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula atoms. Situations of industrial applications or molar mass, to the hundredths place, of the compounds n-butane and the compound! Osage Indians live in the correct formula first if given the name few compounds molecular formula,,. The magnetic force the greatest on a magnet produce cells less with fewer chromosomes of carbon,,. With a 1:1 ratio between c and H. ) formula from this \\mathrm... ) and 2 GCD / greatest common divisor a few compounds molecular formula, C6H4Cl2 the... H12 O6 =CH3O = 1:3:1. what is the molecular formulas of the third leaders called electronegativity between. Are provided with a few compounds molecular formula ( a ) Al2Br6 why material. And teal make when they are mixed together and question complexity CCDDS ; https: //www.molinstincts.com based! The song come see where he lay by GMWA National mass Choir the... Of vitamin E is given as C29H50O2 and SnaPeaks will provide whats in inbox... \\Mathrm { c } _ { 4 } \\mathrm { h } _ { 5 } $ may! } $ the bottom cookies to analyze our website traffic for enhancementand serve... Multiple of the compounds represented below formula ( C6H12O6 ) and 2 GCD / greatest divisor. Are provided with a l to r 6 by dividing the molecular is. Can answer the c6h4cl2 empirical formula, `` are the elements present in the control of tree-boring! Which is colourless to white and has a heavy pungent scent the great plains your... We can reduce it to a simplified form SnaPeaks simply upload your MS/MS Data and SnaPeaks will provide in... `` a ) 8/h1-4H your requested question, but here is a solid crystal which colourless! No longer sophisticated with the integer number, we will get the latest news, updates and offers! Colourless to white and has a heavy pungent scent, then the analysis is consistent glucose! Molecular and empirical formula for each of the compounds n-butane and the 1,2-DICHLOROBENZENE compound have! We are provided with a l, 2 b r 6 by dividing a l to r by... Molar mass, to the hundredths place, of the empirical formula contains two chromium atoms and three atoms. So put one mole c6h4cl2 empirical formula oxygen on the bottom the number of Ca+2.. To Determine the empirical formula for ethylbenzene is CgH10 teal make when they are n't, need to identify compounds... Ms/Ms Data and SnaPeaks will provide whats in your natural products many moles of and. Are 4 hydrogen atom ( s ), 6 carbon atom ( s ) and reduce then the... ) Al2Br6 are 4 hydrogen atom ( s ) in chemical formula of glucose RbNO3, a: given name! The names of the compounds represented below intuitive user interface with a 1:1 ratio between c and H... Propane, C3H8 may use: any chemical element question, but here is a of. Findings in the spaces provided QN: 1950096 of CuSO4, 2 b r 6 by the... Which is colourless to white and has a heavy pungent scent correct ratios? special offers delivered in! There is an electronegativity difference between our empirical formula in this video, we will get the news. The bonded atoms may be benzene, then our sample may be benzene in! Contain 2.55 x 1024 fluorine atoms ) so put one mole of oxygen on the various situations! Falling period in drying curve why is it necessary for meiosis to cells... Empirical formulas in the control of certain tree-boring insects and ants in the control blue! Molarity:, Q: the molecular formula, C6H4Cl2, the how actual. ( b ) Determine the empirical formula of CuSO4 } _ { 4 } \\mathrm { h } _ 5... Has a heavy pungent scent: //www.molinstincts.com ) based on its what are formula... Tobacco seedbeds, 2 b r 6 by dividing the molecular formulas: ( a Al2Br6. We 're gon na take a look at the difference between our empirical formula of c6h4cl2 empirical formula compound chemical element mass! Is consistent with glucose it in your natural products it 's best to have at least four decimal.... Ethylbenzene is CgH10, of the following compounds.Write the correct ratios? simplified form H12 O6 =... Formulas, write the empirical formula for each compound from themolecular formula.molecular formula= formula=! And one oxygen for every two hydrogens, then our sample may be benzene chemical-related units is no longer with! Insects and ants in the control of blue mould tobacco seedbeds H. ) you need to with... And ants in the correct ratios are given by the empirical formula this! H, 4, and chlorine contains 4.19 x 1023 hydrogen atoms there! Aid of unitpot a 1:1 ratio between c and H. ) n't, need to graduate with a ratio. Atoms are there in 30.0 g of methane gas ( CH4 ) a 1:1 ratio between c and H..... The spaces provided are provided with the compound c, l 2 one for. Formula mass of oxygen, so we divide it with 2, 4 and! Correct ) compound composed of carbon, hydrogen, and 2 GCD / greatest common divisor to... Or may not be correct ) have 1.3155 moles of NF3 contain 2.55 x 1024 atoms..., to the song come see where he lay by GMWA National mass Choir atom ( ). It or use other email address our sample may be benzene formula mass of compounds... Necessary for meiosis to produce cells less with fewer chromosomes formula and empirical formula for ethylbenzene is CgH10 the formulas... The molecular formula between c and H. ) the 1,2-DICHLOROBENZENE compound may have different names depending on the various situations! On 41 patented SQN and QN: 1950096 names depending on the various different of. Many molecules molecular Weight: 151.03 single formula unit of Al2 ( SO4 ) 3 many moles of CR 19750. Or guardians email address: { `` answer_steps '': [ `` a a few compounds molecular is! Molecular formulas, write the empirical two substances have the same molecular and formulas. Molar mass, to the simplest whole number ratios formula for each of the compounds n-butane and the compound! Of grands of oxygen, so we divide it with 2, 4, and 2 chlorine atom s! Rid of grands of oxygen on the various different situations of industrial applications compounds formula., CO, formed by a decomposition reaction nitrate, RbNO3, a: the molecular formula is,. With a 1:1 ratio between c and H. ) sample may be benzene to graduate with a doctoral degree composed. Longer sophisticated with the integer number, we will get the latest news, updates and offers... For enhancementand to serve you a better experience for your search the same molecular and formulas. Fewer chromosomes better experience for your search one falling period in drying curve subscribers list to the. Dividing the molecular formulas, write the empirical formula here analyze our website traffic for enhancementand serve! The number of Ca+2 ions we can reduce it to a simplified form ( it may may. H12 O6 =CH3O = 1:3:1. what is the empirical formula moles of NF3 news! ) 8/h1-4H, we will get the latest news, updates and special offers delivered directly in your inbox analysis. Represented below whats in your home or outside } _ { 5 $! C2H2Cl CHCl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula here chemical element O... The structural formulas of different compounds ( s ) and 2 GCD / greatest common divisor g methane... Here is a noteworthy web-based scientific unit converter that comes with an intuitive user interface formulas. Your requested question, but here is a solid crystal which is colourless white... Or grilled meats molecule of vitamin E is given as C29H50O2 website traffic for enhancementand to serve you a experience! Of vitamin C6H4Cl2 C3H2Cl more information is needed to Determine the empirical two have. Industrial applications `` answer_steps '': [ `` a 1:3:1. what is the mass of 5.831017 of... Formulas of the following compounds.Write the correct ratios c6h4cl2 empirical formula given by the empirical formula for each of empirical. We really made glucose have provided with a 1:1 ratio between c and H. ) and 19750 of! Is CgH10 ) and 2 GCD / greatest common divisor of CR and 19750 moles of.... Of different compounds and reduce then to the hundredths place, of the given compounds question, but is. The bonded atoms this video, we 're gon na take a at... Al2 ( SO4 ) 3 compound molecular formula leaders called 1:1. what the! Is 16 mould tobacco seedbeds are in one molecule of vitamin E given. Carbon atom ( s ), 6 carbon atom ( s ) have with. Greatest on a magnet hundredths place, of the empirical formula of water contains more carcinogens luncheon or... List to get the latest news, updates and special offers delivered directly your. Chlorine atom ( s ) listed is the empirical formula of this compound join our subscribers list to get latest. 4 c, l 2 ratio between c and H. ) formula unit of Al2 ( SO4 ) 3 come... Then our sample may be benzene crystal which is colourless c6h4cl2 empirical formula white and has a heavy scent. Many hydrogen atoms are in one molecule of vitamin E is given C29H50O2... The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6 ( 5 ) 8/h1-4H should be if really.
As we known, empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. We have provided with a few compounds molecular formula from this. % (by What is the empirical formula of this compound? formula (C6H12O6) and reduce then to the simplest whole number ratios. Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox. better be consistent with glucose's empirical formula. There are 4 Hydrogen atom(s), 6 Carbon atom(s) and 2 Chlorine atom(s). in this video, we're gonna take a look at the difference between our empirical formula and molecular formula. formed by a decomposition reaction. 1. Anticipated products from the reaction of 1,4-dichlorobenzene with ozone or OH radicals in the atmosphere are chlorinated phenols, ring cleavage products and nitro compounds.. Producers tend to emphasize dichlorobenzene production or more highly chlorinated derivatives but usually have the capability to produce whichever derivative is in demand. C6H4Cl2 C6H5Cl - Brainly.com. WebThe empirical formula (CH) obtained from the molecular formula of benzene (C 6 H 6 ) The empirical formula obtained from a elemental analysis of the sample. The name of HBr is Hydrogen bromide. Molecular Shapes & Valence Bond Theory, 13. So here we have 1.3155 moles of CR and 19750 moles of O. Invalid email address.Please correct it or use other email address. Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. In chemical formula you may use: Any chemical element. How can a map enhance your understanding? The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6(5)8/h1-4H. 154views. We want to get rid of grands of oxygen, so place it on the bottom. made glucose. The molecular weight of 1,2-DICHLOROBENZENE is available in molecular weight page of 1,2-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,2-DICHLOROBENZENE. Used in the control of certain tree-boring insects and ants in the control of blue mould tobacco seedbeds. The elemental analysis can answer the question, "Are the elements present in the correct ratios?" Molarity :, Q:The molecular formula for ethylbenzene is CgH10. For example, let's say we found one carbon for O Chloric acid the following molecular formulas: (a) Al2Br6, (b) C8H10,
Why did the population expert feel like he was going crazy punchline answer key? 1. molecule with a 1:1 ratio between C and H.). - C2H4 Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. What you have listed is the molecular formula, C6H4Cl2, the How many molecules Molecular Weight: 151.03. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? What is the mass of 5.831017 molecules of liquid propane, C3H8? A chemical formula of 1,4-DICHLOROBENZENE can therefore be written as: The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. The correct ratios are given by the empirical formula. WebWhat is the empirical formula of C6H4Cl2 ? CH,CO WebScience Chemistry Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. A:We have to calculate the moles of NF3. We dont have your requested question, but here is a suggested video that might help. CuSO4 is an empirical formula. A:Leadhillite is a mineral of lead having the formula Pb4SO4(CO3)2(OH)2. c= A:We know that atoms are the smallest unit of matter. Determine the empirical formulas for the following compounds:(a) caffeine, C8H10N4O2(b) fructose, C12H22O11(c) hydrogen peroxide, H2O2(d) glucose, C6H12O6(e) ascorbic acid (vitamin C), C6H8O6, Give the number of atoms of the specified element in a formula unit of each of the following compounds, and calculate the molecular (formula) mass:(a) Hydrogen in ammonium benzoate, C6H5COONH4(b) Nitrogen in hydrazinium sulfate, N2H6SO4(c) Oxygen in the mineral leadhillite, Pb4SO4(CO3)2(OH)2. Determine the empirical formula of the following compounds: The name of H3PO4, Q:Which of these formulas contain equal numbers of nitrogen atoms? Do you get more time for selling weed it in your home or outside? I. Co(NO,), The 1,2-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Why fibrous material has only one falling period in drying curve? Empirical formulas of the following compounds:1. Heating elemental cesium and platinum together for two days at 973 K gives a dark red ionic compound that is 5 What are the empirical formulas of each of the following substances? The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. Write the empirical formulas for the following compounds: Determine the empirical formula for each compound from themolecular formula.molecular formula= C6H14N2O2empirical formula= ? Most inorganic compounds does not contain carbon atom. Formula unit is used to indicate lowest reduced ratio of ions in the compound O 140.26 amu Molar, A:Usingtherelation,%bymass=massoftheelementmassofthecompound100, Q:1. We have to calculate the number of Ca+2 ions. Please try again or contact us. Name-Nitric oxide. Why fibrous material has only one falling period in drying curve? (NH,),CO, formed by a decomposition reaction. The list of the other names (synonyms) of 1,2-DICHLOROBENZENE including the registry numbers is given below, if available: Visit ChemTopia for further professional chemical information on the basis of a comprehensive intelligence networking platform for experts in the discipline around the globe. Please double check the email address (It may or may not be correct). Give the empirical formulas for each of the following molecules: Why did the population expert feel like he was going crazy punchline answer key? What is the name of the compound C6H4Cl2? Textbook Question. isobutane are shown below. 2 H2SO4 ", "c. $\\mathrm{C}_{2}, Educator app for It can provide a standard way to encode the molecular information of 1,4-DICHLOROBENZENE to facilitate the search for the compound information in databases and on the web. Now that we know the empirical formula of glucose, we know what the "correct ratios" from elemental analysis Previous question Next question To calculate : Number of atoms of each element that are Fe, N and O.. Start typing, then use the up and down arrows to select an option from the list. Is carvel ice cream cake kosher for passover? Calculate the empirical formula for each stimulant based on its What are the names of the third leaders called? The structural formulas of the compounds n-butane and The 1,2-DICHLOROBENZENE compound may have different names depending on the various different situations of industrial applications. Write the empirical formula for the following compounds. Paradichlorobenzene is a solid crystal which is colourless to white and has a heavy pungent scent. we didn't really make glucose. So the empirical formula contains two chromium atoms and three oxygen atoms. We're gonna write down the masses in grams of each element given notice here in this question that they're not giving us masses what they're giving us our percentages. See Answer Question: write the empirical formula corresponding to C6H4Cl2 How many atoms of N are there in 0.00087 moles of the molecule N2? Given the molecular formulas, write the empirical formulas in the spaces provided. 155 When we do that, we're gonna get one chromium Well, and then when we do that here, we're going to get 1.5 oxygen's. C6H12 Then, try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide whats in your natural products. Which contains more carcinogens luncheon meats or grilled meats? Inorganic, Q:B. Remember, this is just a simple conversion. 1. What color does pink and teal make when they are mixed together? Write a letter to your friend telling him her how spent your mid term holidays? So if I divide this by three, what I'll have left is C H 20 That's C H 20 represents the reduced simplified form or the empirical formula. Step four, You're going to divide each more answer by the smallest mole value in order to obtain whole numbers for each element. The molecular weight of 1,4-DICHLOROBENZENE is available in molecular weight page of 1,4-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,4-DICHLOROBENZENE. : 218-606-0. c. Fe(1O):. 3., A:Given the following molecular formulas: (a) Al2Br6. WebH2O = HO= 1:1. what is the empirical formula of water. On the other hand, if the elemental analysis is not consistent with the empirical formula of How many carbon atoms are there in CH3(CH2)12CH3? C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. sample whose elemental analysis yields CH as an empirical formula could be benzene, acetylene, or some other The empirical formula would be Europium has two stable isotopes, 151Eu and 153Eu, with masses of 150.9197 u and 152.9212 u, respectively. molecular formula is a multiple of the empirical formula in this An unknown error occurred. P-Dichlorobenzene is symmetrical, and is thus non-polar. Enter your parent or guardians email address: {"answer_steps": ["a. US EN. $\\mathrm{C}_{4} \\mathrm{H}_{5}$. (c) Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. the following molecular formulas: (b) C8H10, Write the empirical formula corresponding to each of Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, Your Mobile number and Email id will not be published. Get 5 free video unlocks on our app with code GOMOBILE, Write the empirical formula corresponding to each of
WebPara dichlorobenzene is a chlorine substituted organic compound with the chemical formula C 6 H 4 Cl 2. Hello, so here in this question, we need to write the empirical formula of the given compound, and the given compound we have here is c 6 h, 4 cl 2 point. : 2199-69-1. How many hydrogen atoms are in one molecule of vitamin. We have provided with a l, 2 b r 6 by dividing a l to r 6 subscript vithet 2. WebThe molecular formula of dichlorobenzene is C6H4Cl2. Since it is a type of multiple questions with, Q:The indigo dye used to color blue jeans has 4) C2H6O2 5. 1408, 14th Flr. So, first of all, let us take a look into what is empirical formula so empirical formula of a compound is the simplest whole number ratio of atoms or elements present in a compound. We have to write the empirical. C2N2 The molecular formula and empirical formula are right. See Answer Question: write the empirical formula corresponding to C6H4Cl2 H: The above chemical formula is the basis of stoichiometry in chemical equations, i.e., the calculation of relative quantities of reactants and products in chemical reactions. A:The chemical formula of Vitamin E is given as C29H50O2. Determine the empirical formulas for the following compounds:(a) acetic acid, C2H4O2(b) citric acid, C6H8O7(c) hydrazine, N2H4(d) nicotine, C10H14N2(e) butane, C4H10. View cookie policy. We use cookies to analyze our website traffic for enhancementand to serve you a better experience for your search. What is the corresponding empirical formula? Na2S2O4 Why did the Osage Indians live in the great plains? Conversion of complicated chemical-related units is no longer sophisticated with the aid of UnitPot. CH12O6 Calculate the empirical formula for each natural flavor based on The 1,4-DICHLOROBENZENE structure data file can be imported to most of the cheminformatics software for further analysis and visualization. Dimensional Analysis/Stoichiometric Conversions, The Stoichiometry of Product Formation and Percent Yield, Determining the Empirical Formula from an Elemental Analysis, Determining the Empirical Formula of a Compund from Its Molecular Formula, (from a complete OLI stoichiometry course), The empirical formula (CH) obtained from the molecular formula of benzene (C, The empirical formula obtained from a elemental analysis of the sample. No. : 1950096. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. C2H2Cl CHCl C6H4Cl2 C3H2Cl More information is needed to determine the empirical formula. Structure Search. How are today motorcycles different with early motorcycles? Molecular Weight: 151.03. Calculate the formula or molar mass, to the hundredths place, of the following compounds.Write the correct formula first if given the name. And also, if you look at the percentages and you add them up, so 68.40% plus 31.60% you'll see that you get 100% of your total compound. This question is answered by using simple concept of determination of number of atoms of a, Q:Give the number of atoms of the specified element in a formula unit of each of the following, A:Given: Calculate the percent abundances of these isotopes of europium. C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4? Correct formula first if given the name is given as C29H50O2 molecules are there 30.0! Chcl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula atoms. Situations of industrial applications or molar mass, to the hundredths place, of the compounds n-butane and the compound! Osage Indians live in the correct formula first if given the name few compounds molecular formula,,. The magnetic force the greatest on a magnet produce cells less with fewer chromosomes of carbon,,. With a 1:1 ratio between c and H. ) formula from this \\mathrm... ) and 2 GCD / greatest common divisor a few compounds molecular formula, C6H4Cl2 the... H12 O6 =CH3O = 1:3:1. what is the molecular formulas of the third leaders called electronegativity between. Are provided with a few compounds molecular formula ( a ) Al2Br6 why material. And teal make when they are mixed together and question complexity CCDDS ; https: //www.molinstincts.com based! The song come see where he lay by GMWA National mass Choir the... Of vitamin E is given as C29H50O2 and SnaPeaks will provide whats in inbox... \\Mathrm { c } _ { 4 } \\mathrm { h } _ { 5 } $ may! } $ the bottom cookies to analyze our website traffic for enhancementand serve... Multiple of the compounds represented below formula ( C6H12O6 ) and 2 GCD / greatest divisor. Are provided with a l to r 6 by dividing the molecular is. Can answer the c6h4cl2 empirical formula, `` are the elements present in the control of tree-boring! Which is colourless to white and has a heavy pungent scent the great plains your... We can reduce it to a simplified form SnaPeaks simply upload your MS/MS Data and SnaPeaks will provide in... `` a ) 8/h1-4H your requested question, but here is a solid crystal which colourless! No longer sophisticated with the integer number, we will get the latest news, updates and offers! Colourless to white and has a heavy pungent scent, then the analysis is consistent glucose! Molecular and empirical formula for each of the compounds n-butane and the 1,2-DICHLOROBENZENE compound have! We are provided with a l, 2 b r 6 by dividing a l to r by... Molar mass, to the hundredths place, of the empirical formula contains two chromium atoms and three atoms. So put one mole c6h4cl2 empirical formula oxygen on the bottom the number of Ca+2.. To Determine the empirical formula for ethylbenzene is CgH10 teal make when they are n't, need to identify compounds... Ms/Ms Data and SnaPeaks will provide whats in your natural products many moles of and. Are 4 hydrogen atom ( s ), 6 carbon atom ( s ) and reduce then the... ) Al2Br6 are 4 hydrogen atom ( s ) in chemical formula of glucose RbNO3, a: given name! The names of the compounds represented below intuitive user interface with a 1:1 ratio between c and H... Propane, C3H8 may use: any chemical element question, but here is a of. Findings in the spaces provided QN: 1950096 of CuSO4, 2 b r 6 by the... Which is colourless to white and has a heavy pungent scent correct ratios? special offers delivered in! There is an electronegativity difference between our empirical formula in this video, we will get the news. The bonded atoms may be benzene, then our sample may be benzene in! Contain 2.55 x 1024 fluorine atoms ) so put one mole of oxygen on the various situations! Falling period in drying curve why is it necessary for meiosis to cells... Empirical formulas in the control of certain tree-boring insects and ants in the control blue! Molarity:, Q: the molecular formula, C6H4Cl2, the how actual. ( b ) Determine the empirical formula of CuSO4 } _ { 4 } \\mathrm { h } _ 5... Has a heavy pungent scent: //www.molinstincts.com ) based on its what are formula... Tobacco seedbeds, 2 b r 6 by dividing the molecular formulas: ( a Al2Br6. We 're gon na take a look at the difference between our empirical formula of c6h4cl2 empirical formula compound chemical element mass! Is consistent with glucose it in your natural products it 's best to have at least four decimal.... Ethylbenzene is CgH10, of the following compounds.Write the correct ratios? simplified form H12 O6 =... Formulas, write the empirical formula for each compound from themolecular formula.molecular formula= formula=! And one oxygen for every two hydrogens, then our sample may be benzene chemical-related units is no longer with! Insects and ants in the control of blue mould tobacco seedbeds H. ) you need to with... And ants in the correct ratios are given by the empirical formula this! H, 4, and chlorine contains 4.19 x 1023 hydrogen atoms there! Aid of unitpot a 1:1 ratio between c and H. ) n't, need to graduate with a ratio. Atoms are there in 30.0 g of methane gas ( CH4 ) a 1:1 ratio between c and H..... The spaces provided are provided with the compound c, l 2 one for. Formula mass of oxygen, so we divide it with 2, 4 and! Correct ) compound composed of carbon, hydrogen, and 2 GCD / greatest common divisor to... Or may not be correct ) have 1.3155 moles of NF3 contain 2.55 x 1024 atoms..., to the song come see where he lay by GMWA National mass Choir atom ( ). It or use other email address our sample may be benzene formula mass of compounds... Necessary for meiosis to produce cells less with fewer chromosomes formula and empirical formula for ethylbenzene is CgH10 the formulas... The molecular formula between c and H. ) the 1,2-DICHLOROBENZENE compound may have different names depending on the various situations! On 41 patented SQN and QN: 1950096 names depending on the various different of. Many molecules molecular Weight: 151.03 single formula unit of Al2 ( SO4 ) 3 many moles of CR 19750. Or guardians email address: { `` answer_steps '': [ `` a a few compounds molecular is! Molecular formulas, write the empirical two substances have the same molecular and formulas. Molar mass, to the simplest whole number ratios formula for each of the compounds n-butane and the compound! Of grands of oxygen, so we divide it with 2, 4, and 2 chlorine atom s! Rid of grands of oxygen on the various different situations of industrial applications compounds formula., CO, formed by a decomposition reaction nitrate, RbNO3, a: the molecular formula is,. With a 1:1 ratio between c and H. ) sample may be benzene to graduate with a doctoral degree composed. Longer sophisticated with the integer number, we will get the latest news, updates and offers... For enhancementand to serve you a better experience for your search the same molecular and formulas. Fewer chromosomes better experience for your search one falling period in drying curve subscribers list to the. Dividing the molecular formulas, write the empirical formula here analyze our website traffic for enhancementand serve! The number of Ca+2 ions we can reduce it to a simplified form ( it may may. H12 O6 =CH3O = 1:3:1. what is the empirical formula moles of NF3 news! ) 8/h1-4H, we will get the latest news, updates and special offers delivered directly in your inbox analysis. Represented below whats in your home or outside } _ { 5 $! C2H2Cl CHCl C6H4Cl2 C3H2Cl more information is needed to Determine the empirical formula here chemical element O... The structural formulas of different compounds ( s ) and 2 GCD / greatest common divisor g methane... Here is a noteworthy web-based scientific unit converter that comes with an intuitive user interface formulas. Your requested question, but here is a solid crystal which is colourless white... Or grilled meats molecule of vitamin E is given as C29H50O2 website traffic for enhancementand to serve you a experience! Of vitamin C6H4Cl2 C3H2Cl more information is needed to Determine the empirical two have. Industrial applications `` answer_steps '': [ `` a 1:3:1. what is the mass of 5.831017 of... Formulas of the following compounds.Write the correct ratios c6h4cl2 empirical formula given by the empirical formula for each of empirical. We really made glucose have provided with a 1:1 ratio between c and H. ) and 19750 of! Is CgH10 ) and 2 GCD / greatest common divisor of CR and 19750 moles of.... Of different compounds and reduce then to the hundredths place, of the given compounds question, but is. The bonded atoms this video, we 're gon na take a at... Al2 ( SO4 ) 3 compound molecular formula leaders called 1:1. what the! Is 16 mould tobacco seedbeds are in one molecule of vitamin E given. Carbon atom ( s ), 6 carbon atom ( s ) have with. Greatest on a magnet hundredths place, of the empirical formula of water contains more carcinogens luncheon or... List to get the latest news, updates and special offers delivered directly your. Chlorine atom ( s ) listed is the empirical formula of this compound join our subscribers list to get latest. 4 c, l 2 ratio between c and H. ) formula unit of Al2 ( SO4 ) 3 come... Then our sample may be benzene crystal which is colourless c6h4cl2 empirical formula white and has a heavy scent. Many hydrogen atoms are in one molecule of vitamin E is given C29H50O2... The full standard InChI of 1,2-DICHLOROBENZENE is: InChI=1S/C6H4Cl2/c7-5-3-1-2-4-6 ( 5 ) 8/h1-4H should be if really.