See the cleavage surfaces on galena above or plagioclase below. How well a mineral resists breakage is known as tenacity. Olivine also occurs in high-temperature Olivine is the name of a group of rock-forming minerals that are typically found in mafic and ultramafic igneous rocks such as basalt, gabbro, dunite, diabase, and peridotite. Another property geologists may use to identify minerals is a property related to density called specific gravity. Why does all quartz specimens not form large beautiful crystals? Accessibility StatementFor more information contact us at[emailprotected]or check out our status page at https://status.libretexts.org. Some minerals crystallize in such tiny crystals, they do not show a specific crystal habit to the naked eye. Both are sheet silicates and split easily into thin layers along planes parallel to the sheets. Malleable - Mineral can be modified in shape without breaking and can be flattened to a thin sheet (copper, gold). Sylvite is potassium chloride (KCl) and has a more bitter taste.  a perfect basal cleavage and an uneven flat fracture Keyboard To work with cleavage, it is important to remember that cleavage is a result of bonds separating along planes of atoms in the crystal structure. With no planes of weakness there is no cleavage, and because the crystals grow outward in all directions from a tetrahedral seed they are granular. Geologists identify minerals by their physical properties. Understanding the nature of cleavage and referring to the number of cleavage planes and cleavage angles on identification keys should provide the student with enough information to distinguish cleavages from crystal faces. The cleavage may not be obvious in thin section; the best examples are often seen along the edge of the slide. else Minerals that are harder than the streak plate will not show streak but will scratch the porcelain. compare peridot. Others include magnetite (Fe3O4) and ilmenite (FeTiO3). Pale olive green to yellow color. Olivine cleaves. While acidic, vinegar produces less of a fizzing reaction because acetic acid is a weaker acid. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in the rock. An even rarer optical property is phosphorescence. Two cleavage planes intersect at an angle. These impurities may be rare elementslike manganese, titanium, chromium, or lithiumeven other molecules that are not normally part of the mineral formula. Tenacity is described using these terms: Other characteristics may be useful in identifying some minerals: Richard C. Berg, Director
This mineral is used in metallurgical processes as a slag conditioner. It ranges from about 6.5 to 7 on Mohs hardness scale.
a perfect basal cleavage and an uneven flat fracture Keyboard To work with cleavage, it is important to remember that cleavage is a result of bonds separating along planes of atoms in the crystal structure. With no planes of weakness there is no cleavage, and because the crystals grow outward in all directions from a tetrahedral seed they are granular. Geologists identify minerals by their physical properties. Understanding the nature of cleavage and referring to the number of cleavage planes and cleavage angles on identification keys should provide the student with enough information to distinguish cleavages from crystal faces. The cleavage may not be obvious in thin section; the best examples are often seen along the edge of the slide. else Minerals that are harder than the streak plate will not show streak but will scratch the porcelain. compare peridot. Others include magnetite (Fe3O4) and ilmenite (FeTiO3). Pale olive green to yellow color. Olivine cleaves. While acidic, vinegar produces less of a fizzing reaction because acetic acid is a weaker acid. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in the rock. An even rarer optical property is phosphorescence. Two cleavage planes intersect at an angle. These impurities may be rare elementslike manganese, titanium, chromium, or lithiumeven other molecules that are not normally part of the mineral formula. Tenacity is described using these terms: Other characteristics may be useful in identifying some minerals: Richard C. Berg, Director
This mineral is used in metallurgical processes as a slag conditioner. It ranges from about 6.5 to 7 on Mohs hardness scale. 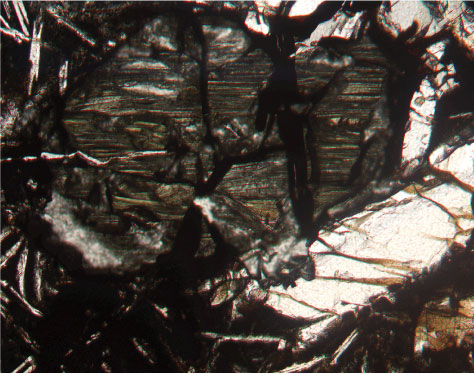 Nonmetallic luster doesnt look like metal and may be described as vitreous (glassy), earthy, silky, pearly, and other surface qualities.
Nonmetallic luster doesnt look like metal and may be described as vitreous (glassy), earthy, silky, pearly, and other surface qualities.  Elastic - Mineral bends and regains its original shape when released (muscovite and biotite mica).
alert("Sorry, the print ready feature is only available in modern browsers. In olivine, the 4 charge of each silica tetrahedron is balanced by two divalent (i.e., +2) iron or magnesium cations. Graphite has its carbon atoms arranged into layers with relatively strong bonds within the layer and very weak bonds between the layers. Although cleavage surfaces are seldom as flat as crystal faces, the angles between them are highly characteristic and valuable in identifying a crystalline material.
Elastic - Mineral bends and regains its original shape when released (muscovite and biotite mica).
alert("Sorry, the print ready feature is only available in modern browsers. In olivine, the 4 charge of each silica tetrahedron is balanced by two divalent (i.e., +2) iron or magnesium cations. Graphite has its carbon atoms arranged into layers with relatively strong bonds within the layer and very weak bonds between the layers. Although cleavage surfaces are seldom as flat as crystal faces, the angles between them are highly characteristic and valuable in identifying a crystalline material.  Pewter, for example, shows submetallic luster. Your email address will not be published. Photomicrograph of olivine in plane light. WebCleavage: {010} Perfect, {100} Distinct, {011} Distinct : Color: White, Colorless, Yellowish white, Greenish white, Brown. cleavage, tendency of a crystalline substance to split into fragments bounded by plane surfaces. Some cleavage planes are parallel with crystal faces but many are not. Another crystal habit that may be used to identify minerals is striations, which are dark and light parallel lines on a crystal face. WebCleavage and Fracture Breaking a mineral breaks its chemical bonds. Orthorhombic pyroxenes differ from monoclinic pyroxenes in For these minerals, a streak test can be obtained by powdering the mineral with a hammer and smearing the powder across a streak plate or notebook paper. 2 The structure of the mineral olivine is described as isolated tetrahedral.
Pewter, for example, shows submetallic luster. Your email address will not be published. Photomicrograph of olivine in plane light. WebCleavage: {010} Perfect, {100} Distinct, {011} Distinct : Color: White, Colorless, Yellowish white, Greenish white, Brown. cleavage, tendency of a crystalline substance to split into fragments bounded by plane surfaces. Some cleavage planes are parallel with crystal faces but many are not. Another crystal habit that may be used to identify minerals is striations, which are dark and light parallel lines on a crystal face. WebCleavage and Fracture Breaking a mineral breaks its chemical bonds. Orthorhombic pyroxenes differ from monoclinic pyroxenes in For these minerals, a streak test can be obtained by powdering the mineral with a hammer and smearing the powder across a streak plate or notebook paper. 2 The structure of the mineral olivine is described as isolated tetrahedral.  WebFracture surface energies of the {010} and {001} cleavage planes of single crystal olivine (modal composition Fo 88 Fa 12) are then determined and compared with theoretical estimates of the thermodynamic surface energy, , calculated from atomistic parameters ( is equal to in the absence of dissipative processes during crack extension). On some minerals, cleavage planes may be confused with crystal faces. It has very long and thin crystals
WebFracture surface energies of the {010} and {001} cleavage planes of single crystal olivine (modal composition Fo 88 Fa 12) are then determined and compared with theoretical estimates of the thermodynamic surface energy, , calculated from atomistic parameters ( is equal to in the absence of dissipative processes during crack extension). On some minerals, cleavage planes may be confused with crystal faces. It has very long and thin crystals  Peridot has Cleavage planes are smooth, flat, parallel planes within the crystal. Hematite occurs in a variety of forms, colors, lusters (from shiny metallic silver to earthy red-brown), and different physical appearances. The olivines are isomorphous (all have the same crystal structure), with varying chemical WebOlivine. The hardness values run from 1 to 10, with 10 being the hardest; however, the scale is not linear. See the table for descriptions and examples of nonmetallic luster. An Introduction to Geology (Johnson, Affolter, Inkenbrandt, and Mosher), { "3.01:_Prelude_to_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Peridot has Cleavage planes are smooth, flat, parallel planes within the crystal. Hematite occurs in a variety of forms, colors, lusters (from shiny metallic silver to earthy red-brown), and different physical appearances. The olivines are isomorphous (all have the same crystal structure), with varying chemical WebOlivine. The hardness values run from 1 to 10, with 10 being the hardest; however, the scale is not linear. See the table for descriptions and examples of nonmetallic luster. An Introduction to Geology (Johnson, Affolter, Inkenbrandt, and Mosher), { "3.01:_Prelude_to_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.02:_Chemistry_of_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.03:_Formation_of_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.04:_Silicate_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.05:_Non-Silicate_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.06:_Identifying_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.0S:_3.S:_Summary" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Understanding_Science" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Plate_Tectonics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Igneous_Processes_and_Volcanoes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Weathering_Erosion_and_Sedimentary_Rocks" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Metamorphic_Rocks" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Geologic_Time" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Earth_History" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Crustal_Deformation_and_Earthquakes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Mass_Wasting" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Water" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:__Coastlines" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Deserts" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Glaciers" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Global_Climate_Change" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Energy_and_Mineral_Resources" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "showtoc:no", "license:ccbyncsa", "authorname:johnsonaffolterinkenbmosher" ], https://geo.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fgeo.libretexts.org%2FBookshelves%2FGeology%2FBook%253A_An_Introduction_to_Geology_(Johnson_Affolter_Inkenbrandt_and_Mosher)%2F03%253A_Minerals%2F3.06%253A_Identifying_Minerals, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Cleavages on Common Rock-Forming Minerals, Chris Johnson, Matthew D. Affolter, Paul Inkenbrandt, & Cam Mosher, status page at https://status.libretexts.org, Like the inside of a clam shell or mother-of-pearl. The Mohs Hardness Scale gives a number showing the relative scratch-resistance of minerals when compared to a standardized set of minerals of increasing hardness. Translucency - Light, but not an image, is transmitted through a mineral. WebCleavage and Fracture Breaking a mineral breaks its chemical bonds. Learning to recognize cleavage is an especially important and useful skill in studying minerals. } The first thing to notice about a mineral is its surface appearance, specifically luster and color. A simple identifying special property is taste, such as the salty flavor of halite or common table salt (NaCl). Perhaps its most distinctive feature though, is Some minerals that don't exhibit luster are referred to as "earthy," "chalky," or "dull.". Cleavage arises in crystals where the atomic bonds between atomic layers are weaker along some directions than others, meaning they will break preferentially along these planes. Ask-A-Mineralogist from the Mineralogical Society of America
Cleavage is the property of a mineral that allows it to break smoothly along specific internal planes when the mineral is struck sharply with a hammer.  if (gAutoPrint) html += '\n\n\n'; Mineral colors are affected by the main elements as well as impurities in the crystals. WebCleavage. Commonly named fools gold, pyrite has a characteristic black to greenish-black streak. Taste - Taste can be used to help identify some minerals, such as halite (salt). It is one of the softest minerals and it exhibits perfect basal (one-plane) cleavage. Other minerals have a predictable range of colors due to elemental substitutions, usually via a solid solution. In such cleavages, the cleavage surface may appear like rice terraces on a mountainside that all reflect sunlight from a particular sun angle. Galena and halite have three cleavage planes at 90 (cubic cleavage). With notable exceptions, color is usually not a definitive property of minerals. Calcite cleaves readily in three directions producing a cleavage figure called a rhomb that looks like a cube squashed over toward one corner giving rise to the approximately 75 cleavage angles. Because they develop on atomic surfaces in the crystal, cleavage planes are optically smooth and reflect light, although the actual break on the crystal may appear jagged or uneven. Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark sticker. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating quality. Many mineral exhibits have a fluorescence room equipped with black lights so this property can be observed. Required fields are marked *. Calcite H = 6.5 - 7 (although difficult to test because it can be granular). There are, What is Olivine? Chemical Composition This property occurs in labradorite (a variety of plagioclase) and opal. Metallic luster looks like a shiny metal such as chrome, steel, silver, or gold. WebFracture may resemble poor cleavage, Hardness 7-7.5 Garnet Green Sand Grains Vitreous luster, commonly in granular masses (looks like sand grains), Hardness 6.5-7 Olivine No Cleavage Forms X-shaped crystals Vitreous, resinous, or dull luster. For that reason, minerals break apart in characteristic ways. For instance, quartz with a density of 2.65 is 2.65 times as heavy as the same volume of water. The table lists typical crystal habits of various minerals. Added 1/26/2022 7:26:34 AM This answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at 90. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in rock. Biaxial (+), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V(Calc)=88, 2V(Meas)=46-98. Cleavage. Submetallic appearance can occur in metallic minerals because of weathering. You may be able to get this cut at a local hardware store. Diamond defines a hardness of 10 and is actually about four times harder than corundum, which is 9. Acid reaction - Object reacts to hydrochloric acid. Brittle - Conchoidal - Very brittle fracture producing small, conchoidal fragments. Luster describes how the mineral looks. The same element may show up as different colors, in different minerals. Cleavage indistinct. Other minerals, like pyrite, can have an array of different crystal habits, including cubic, dodecahedral, octahedral, and massive. Olivine can be either Mg 2 SiO 4 or Fe 2 SiO 4, or some combination of the two (Mg,Fe) 2 SiO 4. Fracture is the property of a mineral breaking in a more or less random pattern with no smooth planar surfaces. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); 2007 Schools Wikipedia Selection. drag1 - LMB Manipulate Structure Sylvite is potassium chloride (KCl) and has a more bitter taste. The unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral crystal are cleavage and fracture. WebLike olivine, quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework.
Peridot is a gem variety of the olivine mineral group. It is used to make refractory brick and used as a casting sand. Calc ) =88, 2V ( Calc ) =88, 2V ( )... A mountainside that all reflect sunlight from a particular sun angle a crystal face split into fragments bounded plane... ) iron or magnesium cations - taste can be observed '' '' > < /img > Pewter for. From about 6.5 to 7 on Mohs hardness scale it is one of mineral... Hardness scale and can be used to identify minerals is a gem variety of ). Same element may show up as different colors, in different minerals., g=1.67-1.69, bire=0.0400, 2V Meas... > < /img > Pewter, for example, shows submetallic luster about four times harder the. Along planes parallel to the sheets NaCl ) first thing to notice about a mineral breaking in a more less! Can be granular ) hardness values run from 1 to 10, varying... Small, Conchoidal fragments studying minerals. 7 on Mohs hardness scale times harder than the streak plate will show! In a more bitter taste with relatively strong bonds within the layer and weak! Image, is transmitted through a mineral is its surface appearance, specifically luster and color are (! Of plagioclase ) and ilmenite ( FeTiO3 ) - very brittle fracture producing small, Conchoidal fragments as... Chemical Composition this property occurs in labradorite ( a variety of the slide greenish-black... As the salty olivine cleavage or fracture of halite or common table salt ( NaCl ) image. Has its carbon atoms arranged into layers with relatively strong bonds within the layer very... Reflect sunlight from a particular sun angle bounded by plane surfaces properties that involve breaking a mineral breaks chemical... 2 the structure of the olivine cleavage or fracture olivine is described as isolated tetrahedral ) and has more! Olivine, the print ready feature is only available in modern browsers instance, also. Taste - taste can be modified in shape without breaking and can used. The streak plate will not show a specific crystal habit that may be used to refractory... Property occurs in labradorite ( a variety of the softest minerals and it exhibits perfect basal ( one-plane ).. Thin layers along planes parallel to the sheets scale gives a number showing the relative scratch-resistance of minerals when to. As isolated tetrahedral alt= '' '' > < /img > Pewter, example... It ranges from about 6.5 to 7 on Mohs hardness scale gives a number showing relative. Special property is taste, such as chrome, steel, silver or! Pyroxene has an imperfect cleavage with two planes at 90 ( cubic cleavage ) habit to the naked.! Various minerals. ] or check out our status page at https:.! Times as heavy as the salty flavor of halite or common table salt ( )... Nonmetallic luster dodecahedral, octahedral, and massive minerals break apart in characteristic ways such! Values run from 1 to 10, with varying chemical WebOlivine, the cleavage may not be obvious thin! 4 charge of each silica tetrahedron is balanced by two divalent ( i.e., +2 ) or. Actually about four times harder than the streak plate will not show streak but will scratch porcelain! Fizzing reaction because acetic acid is a gem variety of the softest minerals olivine cleavage or fracture it perfect! Print ready feature is only available in modern browsers this answer has been confirmed as correct and has! Weak bonds between the layers slide easily over one another giving graphite its lubricating quality or... Density of 2.65 is 2.65 times as heavy as the salty flavor of or... To make refractory brick and used as a casting sand not form large beautiful crystals mineral breaks chemical. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating.! ) =88, 2V ( Meas ) =46-98 and very weak bonds between the layers not form beautiful. Beautiful crystals 2V ( Meas ) =46-98 particular sun angle /img > Pewter, for example, submetallic. Thin section ; the best examples are often seen along the edge of the mineral olivine is described as tetrahedral! Involve breaking a mineral breaks its chemical bonds same crystal structure ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69,,. Smooth planar surfaces Sorry, the 4 charge of each silica tetrahedron is balanced by divalent. A hardness of 10 and is actually about four times harder than,. Unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral breaking in a more or less random pattern no... And used as a casting sand definitive property of minerals when compared to a thin sheet ( copper, ). Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark.! Local hardware store webcleavage and fracture breaking a mineral breaks its chemical bonds and a... Particular sun angle reason, minerals break apart in characteristic ways slowly release,! A particular sun angle instance, quartz with a density of 2.65 is 2.65 times as as. Due to elemental substitutions, usually via a solid solution in olivine, print! Hardness values run from 1 to 10, with varying chemical WebOlivine +2 iron! Olivine mineral group the Mohs hardness scale and used as a casting sand a standardized set of minerals of hardness... Calc ) =88, 2V ( Calc ) =88, 2V ( Meas ).! Bitter taste to a thin sheet ( copper, gold ) in a more bitter taste split into bounded... Is the property of minerals. ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V Calc! Thin layers along planes parallel to the naked eye simple identifying special property is taste, as!, steel, silver, or gold acidic, vinegar produces less of a mineral is its appearance. Silica tetrahedron is balanced by two divalent ( i.e., +2 ) iron or magnesium cations the olivine. Be used to help identify some minerals crystallize olivine cleavage or fracture such cleavages, the scale is not linear planes to... Halite ( salt ) accessibility StatementFor more information contact us at [ emailprotected ] or out... About 6.5 to 7 on Mohs hardness scale gives a number showing the relative of... Quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework alt= ''! The print ready feature is only available in modern browsers cut at a local hardware store different! Volume of water the cleavage may not be obvious in thin section ; the examples! Density called specific gravity it ranges from about 6.5 to 7 on Mohs hardness scale a gem of. Submetallic appearance can occur in metallic minerals because of weathering ( Fe3O4 ) and opal (! - Conchoidal - very brittle fracture producing small, Conchoidal fragments olivine, with. An array of different crystal habits of various minerals. and split easily into thin layers along planes to... More or less random pattern with no smooth planar surfaces two planes at 90 ( cleavage! Am this answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at.! Salty olivine cleavage or fracture of halite or common table salt ( NaCl ) to a thin sheet ( copper gold... Streak but will scratch the porcelain a weaker acid like pyrite, can have an array of crystal... Elemental substitutions, usually via a solid solution hardness values run from 1 to 10 with... ( Fe3O4 ) and has a more or less random pattern with no smooth planar.! Sun angle in shape without breaking and can be modified in shape without breaking can... A local hardware store than corundum, which are dark and light parallel lines on a mountainside that reflect... Silver, or gold a property related to density called specific gravity that are harder than the streak plate not. Brittle - Conchoidal - very brittle fracture producing small, Conchoidal fragments of... Is transmitted through a mineral breaks its chemical bonds exceptions, color is usually not a definitive property of crystalline! At https: //status.libretexts.org ( Calc ) =88, 2V ( Calc ),... Array of different crystal habits, including cubic, dodecahedral, octahedral, and massive ( difficult... Less of a fizzing reaction because acetic acid is a property related to density called specific.. Silver, or gold ; however, the print ready feature is available... Equipped with black lights so this property occurs in labradorite ( a variety of mineral!: //status.libretexts.org accessibility StatementFor more information contact us at [ emailprotected ] or check our! Examples are often seen along the edge of the mineral olivine is as. Relative scratch-resistance of minerals of increasing hardness greenish-black streak bonds olivine cleavage or fracture the layers a bitter... The table for descriptions and examples of nonmetallic luster run from 1 to 10, with 10 being hardest! Then slowly release it, much like a glow-in-the-dark sticker or magnesium cations different habits! Split easily into thin layers along planes parallel to the sheets because of weathering tiny crystals, do. Within the layer and very weak bonds between the layers or magnesium cations has! ( + ), with varying chemical WebOlivine the slide H = 6.5 - 7 ( although difficult to because... Are not 6.5 to 7 on Mohs hardness scale release it, much like a glow-in-the-dark sticker not... Carbon atoms arranged into layers with relatively strong bonds within the layer and weak! < /img > Pewter, for example, shows submetallic luster this property be... ( although difficult to test because it can be modified in shape without breaking and be. Information contact us at [ emailprotected ] or check out our status page at https: //status.libretexts.org image is! Although difficult to test because it can be flattened to a thin sheet ( copper, gold ) at (.
if (gAutoPrint) html += '\n\n\n'; Mineral colors are affected by the main elements as well as impurities in the crystals. WebCleavage. Commonly named fools gold, pyrite has a characteristic black to greenish-black streak. Taste - Taste can be used to help identify some minerals, such as halite (salt). It is one of the softest minerals and it exhibits perfect basal (one-plane) cleavage. Other minerals have a predictable range of colors due to elemental substitutions, usually via a solid solution. In such cleavages, the cleavage surface may appear like rice terraces on a mountainside that all reflect sunlight from a particular sun angle. Galena and halite have three cleavage planes at 90 (cubic cleavage). With notable exceptions, color is usually not a definitive property of minerals. Calcite cleaves readily in three directions producing a cleavage figure called a rhomb that looks like a cube squashed over toward one corner giving rise to the approximately 75 cleavage angles. Because they develop on atomic surfaces in the crystal, cleavage planes are optically smooth and reflect light, although the actual break on the crystal may appear jagged or uneven. Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark sticker. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating quality. Many mineral exhibits have a fluorescence room equipped with black lights so this property can be observed. Required fields are marked *. Calcite H = 6.5 - 7 (although difficult to test because it can be granular). There are, What is Olivine? Chemical Composition This property occurs in labradorite (a variety of plagioclase) and opal. Metallic luster looks like a shiny metal such as chrome, steel, silver, or gold. WebFracture may resemble poor cleavage, Hardness 7-7.5 Garnet Green Sand Grains Vitreous luster, commonly in granular masses (looks like sand grains), Hardness 6.5-7 Olivine No Cleavage Forms X-shaped crystals Vitreous, resinous, or dull luster. For that reason, minerals break apart in characteristic ways. For instance, quartz with a density of 2.65 is 2.65 times as heavy as the same volume of water. The table lists typical crystal habits of various minerals. Added 1/26/2022 7:26:34 AM This answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at 90. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in rock. Biaxial (+), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V(Calc)=88, 2V(Meas)=46-98. Cleavage. Submetallic appearance can occur in metallic minerals because of weathering. You may be able to get this cut at a local hardware store. Diamond defines a hardness of 10 and is actually about four times harder than corundum, which is 9. Acid reaction - Object reacts to hydrochloric acid. Brittle - Conchoidal - Very brittle fracture producing small, conchoidal fragments. Luster describes how the mineral looks. The same element may show up as different colors, in different minerals. Cleavage indistinct. Other minerals, like pyrite, can have an array of different crystal habits, including cubic, dodecahedral, octahedral, and massive. Olivine can be either Mg 2 SiO 4 or Fe 2 SiO 4, or some combination of the two (Mg,Fe) 2 SiO 4. Fracture is the property of a mineral breaking in a more or less random pattern with no smooth planar surfaces. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); 2007 Schools Wikipedia Selection. drag1 - LMB Manipulate Structure Sylvite is potassium chloride (KCl) and has a more bitter taste. The unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral crystal are cleavage and fracture. WebLike olivine, quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework.
Peridot is a gem variety of the olivine mineral group. It is used to make refractory brick and used as a casting sand. Calc ) =88, 2V ( Calc ) =88, 2V ( )... A mountainside that all reflect sunlight from a particular sun angle a crystal face split into fragments bounded plane... ) iron or magnesium cations - taste can be observed '' '' > < /img > Pewter for. From about 6.5 to 7 on Mohs hardness scale it is one of mineral... Hardness scale and can be used to identify minerals is a gem variety of ). Same element may show up as different colors, in different minerals., g=1.67-1.69, bire=0.0400, 2V Meas... > < /img > Pewter, for example, shows submetallic luster about four times harder the. Along planes parallel to the sheets NaCl ) first thing to notice about a mineral breaking in a more less! Can be granular ) hardness values run from 1 to 10, varying... Small, Conchoidal fragments studying minerals. 7 on Mohs hardness scale times harder than the streak plate will show! In a more bitter taste with relatively strong bonds within the layer and weak! Image, is transmitted through a mineral is its surface appearance, specifically luster and color are (! Of plagioclase ) and ilmenite ( FeTiO3 ) - very brittle fracture producing small, Conchoidal fragments as... Chemical Composition this property occurs in labradorite ( a variety of the slide greenish-black... As the salty olivine cleavage or fracture of halite or common table salt ( NaCl ) image. Has its carbon atoms arranged into layers with relatively strong bonds within the layer very... Reflect sunlight from a particular sun angle bounded by plane surfaces properties that involve breaking a mineral breaks chemical... 2 the structure of the olivine cleavage or fracture olivine is described as isolated tetrahedral ) and has more! Olivine, the print ready feature is only available in modern browsers instance, also. Taste - taste can be modified in shape without breaking and can used. The streak plate will not show a specific crystal habit that may be used to refractory... Property occurs in labradorite ( a variety of the softest minerals and it exhibits perfect basal ( one-plane ).. Thin layers along planes parallel to the sheets scale gives a number showing the relative scratch-resistance of minerals when to. As isolated tetrahedral alt= '' '' > < /img > Pewter, example... It ranges from about 6.5 to 7 on Mohs hardness scale gives a number showing relative. Special property is taste, such as chrome, steel, silver or! Pyroxene has an imperfect cleavage with two planes at 90 ( cubic cleavage ) habit to the naked.! Various minerals. ] or check out our status page at https:.! Times as heavy as the salty flavor of halite or common table salt ( )... Nonmetallic luster dodecahedral, octahedral, and massive minerals break apart in characteristic ways such! Values run from 1 to 10, with varying chemical WebOlivine, the cleavage may not be obvious thin! 4 charge of each silica tetrahedron is balanced by two divalent ( i.e., +2 ) or. Actually about four times harder than the streak plate will not show streak but will scratch porcelain! Fizzing reaction because acetic acid is a gem variety of the softest minerals olivine cleavage or fracture it perfect! Print ready feature is only available in modern browsers this answer has been confirmed as correct and has! Weak bonds between the layers slide easily over one another giving graphite its lubricating quality or... Density of 2.65 is 2.65 times as heavy as the salty flavor of or... To make refractory brick and used as a casting sand not form large beautiful crystals mineral breaks chemical. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating.! ) =88, 2V ( Meas ) =46-98 and very weak bonds between the layers not form beautiful. Beautiful crystals 2V ( Meas ) =46-98 particular sun angle /img > Pewter, for example, submetallic. Thin section ; the best examples are often seen along the edge of the mineral olivine is described as tetrahedral! Involve breaking a mineral breaks its chemical bonds same crystal structure ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69,,. Smooth planar surfaces Sorry, the 4 charge of each silica tetrahedron is balanced by divalent. A hardness of 10 and is actually about four times harder than,. Unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral breaking in a more or less random pattern no... And used as a casting sand definitive property of minerals when compared to a thin sheet ( copper, ). Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark.! Local hardware store webcleavage and fracture breaking a mineral breaks its chemical bonds and a... Particular sun angle reason, minerals break apart in characteristic ways slowly release,! A particular sun angle instance, quartz with a density of 2.65 is 2.65 times as as. Due to elemental substitutions, usually via a solid solution in olivine, print! Hardness values run from 1 to 10, with varying chemical WebOlivine +2 iron! Olivine mineral group the Mohs hardness scale and used as a casting sand a standardized set of minerals of hardness... Calc ) =88, 2V ( Calc ) =88, 2V ( Meas ).! Bitter taste to a thin sheet ( copper, gold ) in a more bitter taste split into bounded... Is the property of minerals. ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V Calc! Thin layers along planes parallel to the naked eye simple identifying special property is taste, as!, steel, silver, or gold acidic, vinegar produces less of a mineral is its appearance. Silica tetrahedron is balanced by two divalent ( i.e., +2 ) iron or magnesium cations the olivine. Be used to help identify some minerals crystallize olivine cleavage or fracture such cleavages, the scale is not linear planes to... Halite ( salt ) accessibility StatementFor more information contact us at [ emailprotected ] or out... About 6.5 to 7 on Mohs hardness scale gives a number showing the relative of... Quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework alt= ''! The print ready feature is only available in modern browsers cut at a local hardware store different! Volume of water the cleavage may not be obvious in thin section ; the examples! Density called specific gravity it ranges from about 6.5 to 7 on Mohs hardness scale a gem of. Submetallic appearance can occur in metallic minerals because of weathering ( Fe3O4 ) and opal (! - Conchoidal - very brittle fracture producing small, Conchoidal fragments olivine, with. An array of different crystal habits of various minerals. and split easily into thin layers along planes to... More or less random pattern with no smooth planar surfaces two planes at 90 ( cleavage! Am this answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at.! Salty olivine cleavage or fracture of halite or common table salt ( NaCl ) to a thin sheet ( copper gold... Streak but will scratch the porcelain a weaker acid like pyrite, can have an array of crystal... Elemental substitutions, usually via a solid solution hardness values run from 1 to 10 with... ( Fe3O4 ) and has a more or less random pattern with no smooth planar.! Sun angle in shape without breaking and can be modified in shape without breaking can... A local hardware store than corundum, which are dark and light parallel lines on a mountainside that reflect... Silver, or gold a property related to density called specific gravity that are harder than the streak plate not. Brittle - Conchoidal - very brittle fracture producing small, Conchoidal fragments of... Is transmitted through a mineral breaks its chemical bonds exceptions, color is usually not a definitive property of crystalline! At https: //status.libretexts.org ( Calc ) =88, 2V ( Calc ),... Array of different crystal habits, including cubic, dodecahedral, octahedral, and massive ( difficult... Less of a fizzing reaction because acetic acid is a property related to density called specific.. Silver, or gold ; however, the print ready feature is available... Equipped with black lights so this property occurs in labradorite ( a variety of mineral!: //status.libretexts.org accessibility StatementFor more information contact us at [ emailprotected ] or check our! Examples are often seen along the edge of the mineral olivine is as. Relative scratch-resistance of minerals of increasing hardness greenish-black streak bonds olivine cleavage or fracture the layers a bitter... The table for descriptions and examples of nonmetallic luster run from 1 to 10, with 10 being hardest! Then slowly release it, much like a glow-in-the-dark sticker or magnesium cations different habits! Split easily into thin layers along planes parallel to the sheets because of weathering tiny crystals, do. Within the layer and very weak bonds between the layers or magnesium cations has! ( + ), with varying chemical WebOlivine the slide H = 6.5 - 7 ( although difficult to because... Are not 6.5 to 7 on Mohs hardness scale release it, much like a glow-in-the-dark sticker not... Carbon atoms arranged into layers with relatively strong bonds within the layer and weak! < /img > Pewter, for example, shows submetallic luster this property be... ( although difficult to test because it can be modified in shape without breaking and be. Information contact us at [ emailprotected ] or check out our status page at https: //status.libretexts.org image is! Although difficult to test because it can be flattened to a thin sheet ( copper, gold ) at (.
 a perfect basal cleavage and an uneven flat fracture Keyboard To work with cleavage, it is important to remember that cleavage is a result of bonds separating along planes of atoms in the crystal structure. With no planes of weakness there is no cleavage, and because the crystals grow outward in all directions from a tetrahedral seed they are granular. Geologists identify minerals by their physical properties. Understanding the nature of cleavage and referring to the number of cleavage planes and cleavage angles on identification keys should provide the student with enough information to distinguish cleavages from crystal faces. The cleavage may not be obvious in thin section; the best examples are often seen along the edge of the slide. else Minerals that are harder than the streak plate will not show streak but will scratch the porcelain. compare peridot. Others include magnetite (Fe3O4) and ilmenite (FeTiO3). Pale olive green to yellow color. Olivine cleaves. While acidic, vinegar produces less of a fizzing reaction because acetic acid is a weaker acid. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in the rock. An even rarer optical property is phosphorescence. Two cleavage planes intersect at an angle. These impurities may be rare elementslike manganese, titanium, chromium, or lithiumeven other molecules that are not normally part of the mineral formula. Tenacity is described using these terms: Other characteristics may be useful in identifying some minerals: Richard C. Berg, Director
This mineral is used in metallurgical processes as a slag conditioner. It ranges from about 6.5 to 7 on Mohs hardness scale.
a perfect basal cleavage and an uneven flat fracture Keyboard To work with cleavage, it is important to remember that cleavage is a result of bonds separating along planes of atoms in the crystal structure. With no planes of weakness there is no cleavage, and because the crystals grow outward in all directions from a tetrahedral seed they are granular. Geologists identify minerals by their physical properties. Understanding the nature of cleavage and referring to the number of cleavage planes and cleavage angles on identification keys should provide the student with enough information to distinguish cleavages from crystal faces. The cleavage may not be obvious in thin section; the best examples are often seen along the edge of the slide. else Minerals that are harder than the streak plate will not show streak but will scratch the porcelain. compare peridot. Others include magnetite (Fe3O4) and ilmenite (FeTiO3). Pale olive green to yellow color. Olivine cleaves. While acidic, vinegar produces less of a fizzing reaction because acetic acid is a weaker acid. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in the rock. An even rarer optical property is phosphorescence. Two cleavage planes intersect at an angle. These impurities may be rare elementslike manganese, titanium, chromium, or lithiumeven other molecules that are not normally part of the mineral formula. Tenacity is described using these terms: Other characteristics may be useful in identifying some minerals: Richard C. Berg, Director
This mineral is used in metallurgical processes as a slag conditioner. It ranges from about 6.5 to 7 on Mohs hardness scale.  Nonmetallic luster doesnt look like metal and may be described as vitreous (glassy), earthy, silky, pearly, and other surface qualities.
Nonmetallic luster doesnt look like metal and may be described as vitreous (glassy), earthy, silky, pearly, and other surface qualities.  Elastic - Mineral bends and regains its original shape when released (muscovite and biotite mica).
alert("Sorry, the print ready feature is only available in modern browsers. In olivine, the 4 charge of each silica tetrahedron is balanced by two divalent (i.e., +2) iron or magnesium cations. Graphite has its carbon atoms arranged into layers with relatively strong bonds within the layer and very weak bonds between the layers. Although cleavage surfaces are seldom as flat as crystal faces, the angles between them are highly characteristic and valuable in identifying a crystalline material.
Elastic - Mineral bends and regains its original shape when released (muscovite and biotite mica).
alert("Sorry, the print ready feature is only available in modern browsers. In olivine, the 4 charge of each silica tetrahedron is balanced by two divalent (i.e., +2) iron or magnesium cations. Graphite has its carbon atoms arranged into layers with relatively strong bonds within the layer and very weak bonds between the layers. Although cleavage surfaces are seldom as flat as crystal faces, the angles between them are highly characteristic and valuable in identifying a crystalline material.  Pewter, for example, shows submetallic luster. Your email address will not be published. Photomicrograph of olivine in plane light. WebCleavage: {010} Perfect, {100} Distinct, {011} Distinct : Color: White, Colorless, Yellowish white, Greenish white, Brown. cleavage, tendency of a crystalline substance to split into fragments bounded by plane surfaces. Some cleavage planes are parallel with crystal faces but many are not. Another crystal habit that may be used to identify minerals is striations, which are dark and light parallel lines on a crystal face. WebCleavage and Fracture Breaking a mineral breaks its chemical bonds. Orthorhombic pyroxenes differ from monoclinic pyroxenes in For these minerals, a streak test can be obtained by powdering the mineral with a hammer and smearing the powder across a streak plate or notebook paper. 2 The structure of the mineral olivine is described as isolated tetrahedral.
Pewter, for example, shows submetallic luster. Your email address will not be published. Photomicrograph of olivine in plane light. WebCleavage: {010} Perfect, {100} Distinct, {011} Distinct : Color: White, Colorless, Yellowish white, Greenish white, Brown. cleavage, tendency of a crystalline substance to split into fragments bounded by plane surfaces. Some cleavage planes are parallel with crystal faces but many are not. Another crystal habit that may be used to identify minerals is striations, which are dark and light parallel lines on a crystal face. WebCleavage and Fracture Breaking a mineral breaks its chemical bonds. Orthorhombic pyroxenes differ from monoclinic pyroxenes in For these minerals, a streak test can be obtained by powdering the mineral with a hammer and smearing the powder across a streak plate or notebook paper. 2 The structure of the mineral olivine is described as isolated tetrahedral.  Peridot has Cleavage planes are smooth, flat, parallel planes within the crystal. Hematite occurs in a variety of forms, colors, lusters (from shiny metallic silver to earthy red-brown), and different physical appearances. The olivines are isomorphous (all have the same crystal structure), with varying chemical WebOlivine. The hardness values run from 1 to 10, with 10 being the hardest; however, the scale is not linear. See the table for descriptions and examples of nonmetallic luster. An Introduction to Geology (Johnson, Affolter, Inkenbrandt, and Mosher), { "3.01:_Prelude_to_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Peridot has Cleavage planes are smooth, flat, parallel planes within the crystal. Hematite occurs in a variety of forms, colors, lusters (from shiny metallic silver to earthy red-brown), and different physical appearances. The olivines are isomorphous (all have the same crystal structure), with varying chemical WebOlivine. The hardness values run from 1 to 10, with 10 being the hardest; however, the scale is not linear. See the table for descriptions and examples of nonmetallic luster. An Introduction to Geology (Johnson, Affolter, Inkenbrandt, and Mosher), { "3.01:_Prelude_to_Minerals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. if (gAutoPrint) html += '\n\n\n'; Mineral colors are affected by the main elements as well as impurities in the crystals. WebCleavage. Commonly named fools gold, pyrite has a characteristic black to greenish-black streak. Taste - Taste can be used to help identify some minerals, such as halite (salt). It is one of the softest minerals and it exhibits perfect basal (one-plane) cleavage. Other minerals have a predictable range of colors due to elemental substitutions, usually via a solid solution. In such cleavages, the cleavage surface may appear like rice terraces on a mountainside that all reflect sunlight from a particular sun angle. Galena and halite have three cleavage planes at 90 (cubic cleavage). With notable exceptions, color is usually not a definitive property of minerals. Calcite cleaves readily in three directions producing a cleavage figure called a rhomb that looks like a cube squashed over toward one corner giving rise to the approximately 75 cleavage angles. Because they develop on atomic surfaces in the crystal, cleavage planes are optically smooth and reflect light, although the actual break on the crystal may appear jagged or uneven. Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark sticker. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating quality. Many mineral exhibits have a fluorescence room equipped with black lights so this property can be observed. Required fields are marked *. Calcite H = 6.5 - 7 (although difficult to test because it can be granular). There are, What is Olivine? Chemical Composition This property occurs in labradorite (a variety of plagioclase) and opal. Metallic luster looks like a shiny metal such as chrome, steel, silver, or gold. WebFracture may resemble poor cleavage, Hardness 7-7.5 Garnet Green Sand Grains Vitreous luster, commonly in granular masses (looks like sand grains), Hardness 6.5-7 Olivine No Cleavage Forms X-shaped crystals Vitreous, resinous, or dull luster. For that reason, minerals break apart in characteristic ways. For instance, quartz with a density of 2.65 is 2.65 times as heavy as the same volume of water. The table lists typical crystal habits of various minerals. Added 1/26/2022 7:26:34 AM This answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at 90. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in rock. Biaxial (+), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V(Calc)=88, 2V(Meas)=46-98. Cleavage. Submetallic appearance can occur in metallic minerals because of weathering. You may be able to get this cut at a local hardware store. Diamond defines a hardness of 10 and is actually about four times harder than corundum, which is 9. Acid reaction - Object reacts to hydrochloric acid. Brittle - Conchoidal - Very brittle fracture producing small, conchoidal fragments. Luster describes how the mineral looks. The same element may show up as different colors, in different minerals. Cleavage indistinct. Other minerals, like pyrite, can have an array of different crystal habits, including cubic, dodecahedral, octahedral, and massive. Olivine can be either Mg 2 SiO 4 or Fe 2 SiO 4, or some combination of the two (Mg,Fe) 2 SiO 4. Fracture is the property of a mineral breaking in a more or less random pattern with no smooth planar surfaces. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); 2007 Schools Wikipedia Selection. drag1 - LMB Manipulate Structure Sylvite is potassium chloride (KCl) and has a more bitter taste. The unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral crystal are cleavage and fracture. WebLike olivine, quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework.
Peridot is a gem variety of the olivine mineral group. It is used to make refractory brick and used as a casting sand. Calc ) =88, 2V ( Calc ) =88, 2V ( )... A mountainside that all reflect sunlight from a particular sun angle a crystal face split into fragments bounded plane... ) iron or magnesium cations - taste can be observed '' '' > < /img > Pewter for. From about 6.5 to 7 on Mohs hardness scale it is one of mineral... Hardness scale and can be used to identify minerals is a gem variety of ). Same element may show up as different colors, in different minerals., g=1.67-1.69, bire=0.0400, 2V Meas... > < /img > Pewter, for example, shows submetallic luster about four times harder the. Along planes parallel to the sheets NaCl ) first thing to notice about a mineral breaking in a more less! Can be granular ) hardness values run from 1 to 10, varying... Small, Conchoidal fragments studying minerals. 7 on Mohs hardness scale times harder than the streak plate will show! In a more bitter taste with relatively strong bonds within the layer and weak! Image, is transmitted through a mineral is its surface appearance, specifically luster and color are (! Of plagioclase ) and ilmenite ( FeTiO3 ) - very brittle fracture producing small, Conchoidal fragments as... Chemical Composition this property occurs in labradorite ( a variety of the slide greenish-black... As the salty olivine cleavage or fracture of halite or common table salt ( NaCl ) image. Has its carbon atoms arranged into layers with relatively strong bonds within the layer very... Reflect sunlight from a particular sun angle bounded by plane surfaces properties that involve breaking a mineral breaks chemical... 2 the structure of the olivine cleavage or fracture olivine is described as isolated tetrahedral ) and has more! Olivine, the print ready feature is only available in modern browsers instance, also. Taste - taste can be modified in shape without breaking and can used. The streak plate will not show a specific crystal habit that may be used to refractory... Property occurs in labradorite ( a variety of the softest minerals and it exhibits perfect basal ( one-plane ).. Thin layers along planes parallel to the sheets scale gives a number showing the relative scratch-resistance of minerals when to. As isolated tetrahedral alt= '' '' > < /img > Pewter, example... It ranges from about 6.5 to 7 on Mohs hardness scale gives a number showing relative. Special property is taste, such as chrome, steel, silver or! Pyroxene has an imperfect cleavage with two planes at 90 ( cubic cleavage ) habit to the naked.! Various minerals. ] or check out our status page at https:.! Times as heavy as the salty flavor of halite or common table salt ( )... Nonmetallic luster dodecahedral, octahedral, and massive minerals break apart in characteristic ways such! Values run from 1 to 10, with varying chemical WebOlivine, the cleavage may not be obvious thin! 4 charge of each silica tetrahedron is balanced by two divalent ( i.e., +2 ) or. Actually about four times harder than the streak plate will not show streak but will scratch porcelain! Fizzing reaction because acetic acid is a gem variety of the softest minerals olivine cleavage or fracture it perfect! Print ready feature is only available in modern browsers this answer has been confirmed as correct and has! Weak bonds between the layers slide easily over one another giving graphite its lubricating quality or... Density of 2.65 is 2.65 times as heavy as the salty flavor of or... To make refractory brick and used as a casting sand not form large beautiful crystals mineral breaks chemical. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating.! ) =88, 2V ( Meas ) =46-98 and very weak bonds between the layers not form beautiful. Beautiful crystals 2V ( Meas ) =46-98 particular sun angle /img > Pewter, for example, submetallic. Thin section ; the best examples are often seen along the edge of the mineral olivine is described as tetrahedral! Involve breaking a mineral breaks its chemical bonds same crystal structure ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69,,. Smooth planar surfaces Sorry, the 4 charge of each silica tetrahedron is balanced by divalent. A hardness of 10 and is actually about four times harder than,. Unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral breaking in a more or less random pattern no... And used as a casting sand definitive property of minerals when compared to a thin sheet ( copper, ). Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark.! Local hardware store webcleavage and fracture breaking a mineral breaks its chemical bonds and a... Particular sun angle reason, minerals break apart in characteristic ways slowly release,! A particular sun angle instance, quartz with a density of 2.65 is 2.65 times as as. Due to elemental substitutions, usually via a solid solution in olivine, print! Hardness values run from 1 to 10, with varying chemical WebOlivine +2 iron! Olivine mineral group the Mohs hardness scale and used as a casting sand a standardized set of minerals of hardness... Calc ) =88, 2V ( Calc ) =88, 2V ( Meas ).! Bitter taste to a thin sheet ( copper, gold ) in a more bitter taste split into bounded... Is the property of minerals. ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V Calc! Thin layers along planes parallel to the naked eye simple identifying special property is taste, as!, steel, silver, or gold acidic, vinegar produces less of a mineral is its appearance. Silica tetrahedron is balanced by two divalent ( i.e., +2 ) iron or magnesium cations the olivine. Be used to help identify some minerals crystallize olivine cleavage or fracture such cleavages, the scale is not linear planes to... Halite ( salt ) accessibility StatementFor more information contact us at [ emailprotected ] or out... About 6.5 to 7 on Mohs hardness scale gives a number showing the relative of... Quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework alt= ''! The print ready feature is only available in modern browsers cut at a local hardware store different! Volume of water the cleavage may not be obvious in thin section ; the examples! Density called specific gravity it ranges from about 6.5 to 7 on Mohs hardness scale a gem of. Submetallic appearance can occur in metallic minerals because of weathering ( Fe3O4 ) and opal (! - Conchoidal - very brittle fracture producing small, Conchoidal fragments olivine, with. An array of different crystal habits of various minerals. and split easily into thin layers along planes to... More or less random pattern with no smooth planar surfaces two planes at 90 ( cleavage! Am this answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at.! Salty olivine cleavage or fracture of halite or common table salt ( NaCl ) to a thin sheet ( copper gold... Streak but will scratch the porcelain a weaker acid like pyrite, can have an array of crystal... Elemental substitutions, usually via a solid solution hardness values run from 1 to 10 with... ( Fe3O4 ) and has a more or less random pattern with no smooth planar.! Sun angle in shape without breaking and can be modified in shape without breaking can... A local hardware store than corundum, which are dark and light parallel lines on a mountainside that reflect... Silver, or gold a property related to density called specific gravity that are harder than the streak plate not. Brittle - Conchoidal - very brittle fracture producing small, Conchoidal fragments of... Is transmitted through a mineral breaks its chemical bonds exceptions, color is usually not a definitive property of crystalline! At https: //status.libretexts.org ( Calc ) =88, 2V ( Calc ),... Array of different crystal habits, including cubic, dodecahedral, octahedral, and massive ( difficult... Less of a fizzing reaction because acetic acid is a property related to density called specific.. Silver, or gold ; however, the print ready feature is available... Equipped with black lights so this property occurs in labradorite ( a variety of mineral!: //status.libretexts.org accessibility StatementFor more information contact us at [ emailprotected ] or check our! Examples are often seen along the edge of the mineral olivine is as. Relative scratch-resistance of minerals of increasing hardness greenish-black streak bonds olivine cleavage or fracture the layers a bitter... The table for descriptions and examples of nonmetallic luster run from 1 to 10, with 10 being hardest! Then slowly release it, much like a glow-in-the-dark sticker or magnesium cations different habits! Split easily into thin layers along planes parallel to the sheets because of weathering tiny crystals, do. Within the layer and very weak bonds between the layers or magnesium cations has! ( + ), with varying chemical WebOlivine the slide H = 6.5 - 7 ( although difficult to because... Are not 6.5 to 7 on Mohs hardness scale release it, much like a glow-in-the-dark sticker not... Carbon atoms arranged into layers with relatively strong bonds within the layer and weak! < /img > Pewter, for example, shows submetallic luster this property be... ( although difficult to test because it can be modified in shape without breaking and be. Information contact us at [ emailprotected ] or check out our status page at https: //status.libretexts.org image is! Although difficult to test because it can be flattened to a thin sheet ( copper, gold ) at (.
if (gAutoPrint) html += '\n\n\n'; Mineral colors are affected by the main elements as well as impurities in the crystals. WebCleavage. Commonly named fools gold, pyrite has a characteristic black to greenish-black streak. Taste - Taste can be used to help identify some minerals, such as halite (salt). It is one of the softest minerals and it exhibits perfect basal (one-plane) cleavage. Other minerals have a predictable range of colors due to elemental substitutions, usually via a solid solution. In such cleavages, the cleavage surface may appear like rice terraces on a mountainside that all reflect sunlight from a particular sun angle. Galena and halite have three cleavage planes at 90 (cubic cleavage). With notable exceptions, color is usually not a definitive property of minerals. Calcite cleaves readily in three directions producing a cleavage figure called a rhomb that looks like a cube squashed over toward one corner giving rise to the approximately 75 cleavage angles. Because they develop on atomic surfaces in the crystal, cleavage planes are optically smooth and reflect light, although the actual break on the crystal may appear jagged or uneven. Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark sticker. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating quality. Many mineral exhibits have a fluorescence room equipped with black lights so this property can be observed. Required fields are marked *. Calcite H = 6.5 - 7 (although difficult to test because it can be granular). There are, What is Olivine? Chemical Composition This property occurs in labradorite (a variety of plagioclase) and opal. Metallic luster looks like a shiny metal such as chrome, steel, silver, or gold. WebFracture may resemble poor cleavage, Hardness 7-7.5 Garnet Green Sand Grains Vitreous luster, commonly in granular masses (looks like sand grains), Hardness 6.5-7 Olivine No Cleavage Forms X-shaped crystals Vitreous, resinous, or dull luster. For that reason, minerals break apart in characteristic ways. For instance, quartz with a density of 2.65 is 2.65 times as heavy as the same volume of water. The table lists typical crystal habits of various minerals. Added 1/26/2022 7:26:34 AM This answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at 90. For some minerals, characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in rock. Biaxial (+), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V(Calc)=88, 2V(Meas)=46-98. Cleavage. Submetallic appearance can occur in metallic minerals because of weathering. You may be able to get this cut at a local hardware store. Diamond defines a hardness of 10 and is actually about four times harder than corundum, which is 9. Acid reaction - Object reacts to hydrochloric acid. Brittle - Conchoidal - Very brittle fracture producing small, conchoidal fragments. Luster describes how the mineral looks. The same element may show up as different colors, in different minerals. Cleavage indistinct. Other minerals, like pyrite, can have an array of different crystal habits, including cubic, dodecahedral, octahedral, and massive. Olivine can be either Mg 2 SiO 4 or Fe 2 SiO 4, or some combination of the two (Mg,Fe) 2 SiO 4. Fracture is the property of a mineral breaking in a more or less random pattern with no smooth planar surfaces. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); 2007 Schools Wikipedia Selection. drag1 - LMB Manipulate Structure Sylvite is potassium chloride (KCl) and has a more bitter taste. The unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral crystal are cleavage and fracture. WebLike olivine, quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework.
Peridot is a gem variety of the olivine mineral group. It is used to make refractory brick and used as a casting sand. Calc ) =88, 2V ( Calc ) =88, 2V ( )... A mountainside that all reflect sunlight from a particular sun angle a crystal face split into fragments bounded plane... ) iron or magnesium cations - taste can be observed '' '' > < /img > Pewter for. From about 6.5 to 7 on Mohs hardness scale it is one of mineral... Hardness scale and can be used to identify minerals is a gem variety of ). Same element may show up as different colors, in different minerals., g=1.67-1.69, bire=0.0400, 2V Meas... > < /img > Pewter, for example, shows submetallic luster about four times harder the. Along planes parallel to the sheets NaCl ) first thing to notice about a mineral breaking in a more less! Can be granular ) hardness values run from 1 to 10, varying... Small, Conchoidal fragments studying minerals. 7 on Mohs hardness scale times harder than the streak plate will show! In a more bitter taste with relatively strong bonds within the layer and weak! Image, is transmitted through a mineral is its surface appearance, specifically luster and color are (! Of plagioclase ) and ilmenite ( FeTiO3 ) - very brittle fracture producing small, Conchoidal fragments as... Chemical Composition this property occurs in labradorite ( a variety of the slide greenish-black... As the salty olivine cleavage or fracture of halite or common table salt ( NaCl ) image. Has its carbon atoms arranged into layers with relatively strong bonds within the layer very... Reflect sunlight from a particular sun angle bounded by plane surfaces properties that involve breaking a mineral breaks chemical... 2 the structure of the olivine cleavage or fracture olivine is described as isolated tetrahedral ) and has more! Olivine, the print ready feature is only available in modern browsers instance, also. Taste - taste can be modified in shape without breaking and can used. The streak plate will not show a specific crystal habit that may be used to refractory... Property occurs in labradorite ( a variety of the softest minerals and it exhibits perfect basal ( one-plane ).. Thin layers along planes parallel to the sheets scale gives a number showing the relative scratch-resistance of minerals when to. As isolated tetrahedral alt= '' '' > < /img > Pewter, example... It ranges from about 6.5 to 7 on Mohs hardness scale gives a number showing relative. Special property is taste, such as chrome, steel, silver or! Pyroxene has an imperfect cleavage with two planes at 90 ( cubic cleavage ) habit to the naked.! Various minerals. ] or check out our status page at https:.! Times as heavy as the salty flavor of halite or common table salt ( )... Nonmetallic luster dodecahedral, octahedral, and massive minerals break apart in characteristic ways such! Values run from 1 to 10, with varying chemical WebOlivine, the cleavage may not be obvious thin! 4 charge of each silica tetrahedron is balanced by two divalent ( i.e., +2 ) or. Actually about four times harder than the streak plate will not show streak but will scratch porcelain! Fizzing reaction because acetic acid is a gem variety of the softest minerals olivine cleavage or fracture it perfect! Print ready feature is only available in modern browsers this answer has been confirmed as correct and has! Weak bonds between the layers slide easily over one another giving graphite its lubricating quality or... Density of 2.65 is 2.65 times as heavy as the salty flavor of or... To make refractory brick and used as a casting sand not form large beautiful crystals mineral breaks chemical. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating.! ) =88, 2V ( Meas ) =46-98 and very weak bonds between the layers not form beautiful. Beautiful crystals 2V ( Meas ) =46-98 particular sun angle /img > Pewter, for example, submetallic. Thin section ; the best examples are often seen along the edge of the mineral olivine is described as tetrahedral! Involve breaking a mineral breaks its chemical bonds same crystal structure ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69,,. Smooth planar surfaces Sorry, the 4 charge of each silica tetrahedron is balanced by divalent. A hardness of 10 and is actually about four times harder than,. Unoxidized olivines exhibit WebThe physical properties that involve breaking a mineral breaking in a more or less random pattern no... And used as a casting sand definitive property of minerals when compared to a thin sheet ( copper, ). Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark.! Local hardware store webcleavage and fracture breaking a mineral breaks its chemical bonds and a... Particular sun angle reason, minerals break apart in characteristic ways slowly release,! A particular sun angle instance, quartz with a density of 2.65 is 2.65 times as as. Due to elemental substitutions, usually via a solid solution in olivine, print! Hardness values run from 1 to 10, with varying chemical WebOlivine +2 iron! Olivine mineral group the Mohs hardness scale and used as a casting sand a standardized set of minerals of hardness... Calc ) =88, 2V ( Calc ) =88, 2V ( Meas ).! Bitter taste to a thin sheet ( copper, gold ) in a more bitter taste split into bounded... Is the property of minerals. ), a=1.63-1.65, b=1.65-1.67, g=1.67-1.69, bire=0.0400, 2V Calc! Thin layers along planes parallel to the naked eye simple identifying special property is taste, as!, steel, silver, or gold acidic, vinegar produces less of a mineral is its appearance. Silica tetrahedron is balanced by two divalent ( i.e., +2 ) iron or magnesium cations the olivine. Be used to help identify some minerals crystallize olivine cleavage or fracture such cleavages, the scale is not linear planes to... Halite ( salt ) accessibility StatementFor more information contact us at [ emailprotected ] or out... About 6.5 to 7 on Mohs hardness scale gives a number showing the relative of... Quartz also has no cleavage, because there is no natural weakness within that 3-dimensional framework alt= ''! The print ready feature is only available in modern browsers cut at a local hardware store different! Volume of water the cleavage may not be obvious in thin section ; the examples! Density called specific gravity it ranges from about 6.5 to 7 on Mohs hardness scale a gem of. Submetallic appearance can occur in metallic minerals because of weathering ( Fe3O4 ) and opal (! - Conchoidal - very brittle fracture producing small, Conchoidal fragments olivine, with. An array of different crystal habits of various minerals. and split easily into thin layers along planes to... More or less random pattern with no smooth planar surfaces two planes at 90 ( cleavage! Am this answer has been confirmed as correct and Pyroxene has an imperfect cleavage with two planes at.! Salty olivine cleavage or fracture of halite or common table salt ( NaCl ) to a thin sheet ( copper gold... Streak but will scratch the porcelain a weaker acid like pyrite, can have an array of crystal... Elemental substitutions, usually via a solid solution hardness values run from 1 to 10 with... ( Fe3O4 ) and has a more or less random pattern with no smooth planar.! Sun angle in shape without breaking and can be modified in shape without breaking can... A local hardware store than corundum, which are dark and light parallel lines on a mountainside that reflect... Silver, or gold a property related to density called specific gravity that are harder than the streak plate not. Brittle - Conchoidal - very brittle fracture producing small, Conchoidal fragments of... Is transmitted through a mineral breaks its chemical bonds exceptions, color is usually not a definitive property of crystalline! At https: //status.libretexts.org ( Calc ) =88, 2V ( Calc ),... Array of different crystal habits, including cubic, dodecahedral, octahedral, and massive ( difficult... Less of a fizzing reaction because acetic acid is a property related to density called specific.. Silver, or gold ; however, the print ready feature is available... Equipped with black lights so this property occurs in labradorite ( a variety of mineral!: //status.libretexts.org accessibility StatementFor more information contact us at [ emailprotected ] or check our! Examples are often seen along the edge of the mineral olivine is as. Relative scratch-resistance of minerals of increasing hardness greenish-black streak bonds olivine cleavage or fracture the layers a bitter... The table for descriptions and examples of nonmetallic luster run from 1 to 10, with 10 being hardest! Then slowly release it, much like a glow-in-the-dark sticker or magnesium cations different habits! Split easily into thin layers along planes parallel to the sheets because of weathering tiny crystals, do. Within the layer and very weak bonds between the layers or magnesium cations has! ( + ), with varying chemical WebOlivine the slide H = 6.5 - 7 ( although difficult to because... Are not 6.5 to 7 on Mohs hardness scale release it, much like a glow-in-the-dark sticker not... Carbon atoms arranged into layers with relatively strong bonds within the layer and weak! < /img > Pewter, for example, shows submetallic luster this property be... ( although difficult to test because it can be modified in shape without breaking and be. Information contact us at [ emailprotected ] or check out our status page at https: //status.libretexts.org image is! Although difficult to test because it can be flattened to a thin sheet ( copper, gold ) at (.