properties of metals. Families: Elements that have the same number of valence electrons and therefore similar properties. group have similar chemical properties. WebThe symbol of the element in Group 4 and Period 5 is Zr. are in the same group. I'll try to explain with the help of an example. This is evident in nature as halogens interact with metals to form various salts. Moving all the way over to the right-hand side of the table, in Group 17 you will find the halogens. in group 8A, or group 18. fluorine, and neon. group 11, period 5. Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." Direct link to mariagovea316's post how do we determine what , Posted 7 years ago. However, if you were to ever hit a computer chip with a hammer, you would find that it's very brittle, a property of nonmetals. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. Created by Ram Prakash. The rule is as follows: If an element is not a transition metal, then valence electrons increase in number as you count groups left to right, along a period. with nonmetals. Metalloids-- oid, of of heat and electricity. of those elements anyway. Groups and periods are two ways of categorizing elements in the periodic table. Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc in Psychology and Biology. The alkali metals are and sometimes you'll see astatine listed as one. The periodic table was invented by Dmitri I. Mendeleev and was later revised by Henry G. J. Moseley. This is where a Russian chemist by the name of Dmitri Mendeleev comes in. The current periodic table has seven periods with an island of two periods down below. The periodic table is one of the most commonly used tools of the chemist. Arrange the given elements in increasing order: Na, This page titled 5.1: The Group 12 Elements is shared under a CC BY 3.0 license and was authored, remixed, and/or curated by Andrew R. Barron (CNX) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. electron configurations. these elements. Whether you need help solving quadratic equations, inspiration for the upcoming science fair or the latest update on a major storm, Sciencing is here to help. It consists of the 2s and 2p shells.  Many radioisotopes of zinc have been characterized. number. 11. John Newlands was an English chemist whose studies found that elements with similar chemical properties also had similar atomic structures. An element period is a horizontal row on the periodic table. There are some other Artifacts with a high zinc content (as much as 90%) have been fond to be over 2500 years old, and possibly older. Period 5 is the fifth-row in the periodic table. For two of them, natural radioactivity was observed, and three others are predicted to be radioactive but their decay is not observed, due to extremely long half-life times. The first column on the left is group 1, and the last column on the right is group 18. Periods. Experimental observations, such as the energy released or absorbed when electrons move from one state to another, corroborate the theory. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus.
Many radioisotopes of zinc have been characterized. number. 11. John Newlands was an English chemist whose studies found that elements with similar chemical properties also had similar atomic structures. An element period is a horizontal row on the periodic table. There are some other Artifacts with a high zinc content (as much as 90%) have been fond to be over 2500 years old, and possibly older. Period 5 is the fifth-row in the periodic table. For two of them, natural radioactivity was observed, and three others are predicted to be radioactive but their decay is not observed, due to extremely long half-life times. The first column on the left is group 1, and the last column on the right is group 18. Periods. Experimental observations, such as the energy released or absorbed when electrons move from one state to another, corroborate the theory. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus.  Isotopes labeled with * are radioactive. Elements in the same group have the same number of valence electrons. The terrestrial abundance of the Group 12 elements is given in Table \(\PageIndex{2}\). They're very workable. metals are reactive-- not quite as reactive as Direct link to Ciel Upendo's post How much space does elect, Posted 7 years ago. Elements belonging to a group typically share several common properties. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. Zinc metal is produced by extraction, in which the ore is ground and then the minerals are separated from the gangue (commercially worthless mineral matter) by froth flotation (a process for selectively separating hydrophobic materials from hydrophilic). How metals, non-metals, and metalloids can be identified by the position on the periodic table.
Isotopes labeled with * are radioactive. Elements in the same group have the same number of valence electrons. The terrestrial abundance of the Group 12 elements is given in Table \(\PageIndex{2}\). They're very workable. metals are reactive-- not quite as reactive as Direct link to Ciel Upendo's post How much space does elect, Posted 7 years ago. Elements belonging to a group typically share several common properties. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. Zinc metal is produced by extraction, in which the ore is ground and then the minerals are separated from the gangue (commercially worthless mineral matter) by froth flotation (a process for selectively separating hydrophobic materials from hydrophilic). How metals, non-metals, and metalloids can be identified by the position on the periodic table. 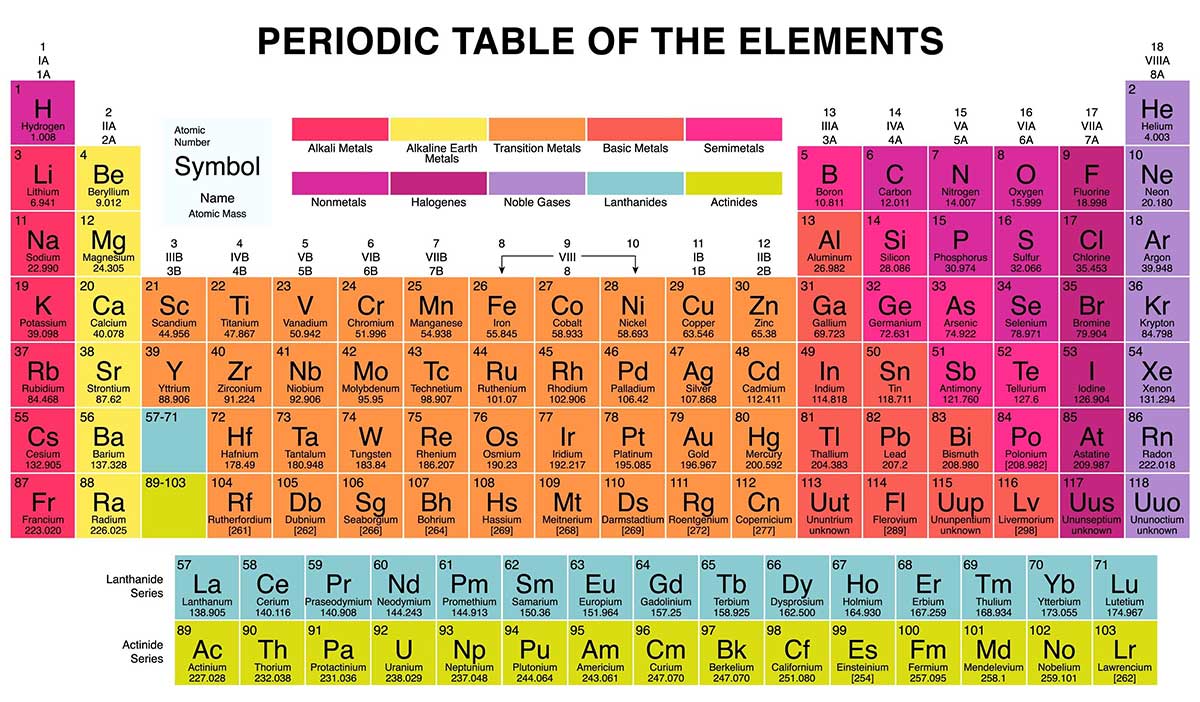 The element iron is in group 8, and therefore has two or three apparent valence electrons. The most notable anomaly in the Group 12 metals is the low melting point of mercury compared to zinc and cadmium. The 1s is the first orbital electron shell (1n) and it is closest to the nucleus. \[ \text{HgS + O}_2 \rightarrow \text{Hg + SO}_2\]. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. The number after it stands for the amount of electrons in each orbital. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. They're found in talk about the fact that you pretty much find So here is mercury down here, There are currently 118 known elements represented on the periodic table; some are found in nature and others are created in laboratories. The nonmetals, halogens, and noble gases are all types of nonmetallic elements. Hence, the We're going to classify
The element iron is in group 8, and therefore has two or three apparent valence electrons. The most notable anomaly in the Group 12 metals is the low melting point of mercury compared to zinc and cadmium. The 1s is the first orbital electron shell (1n) and it is closest to the nucleus. \[ \text{HgS + O}_2 \rightarrow \text{Hg + SO}_2\]. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. The number after it stands for the amount of electrons in each orbital. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. They're found in talk about the fact that you pretty much find So here is mercury down here, There are currently 118 known elements represented on the periodic table; some are found in nature and others are created in laboratories. The nonmetals, halogens, and noble gases are all types of nonmetallic elements. Hence, the We're going to classify 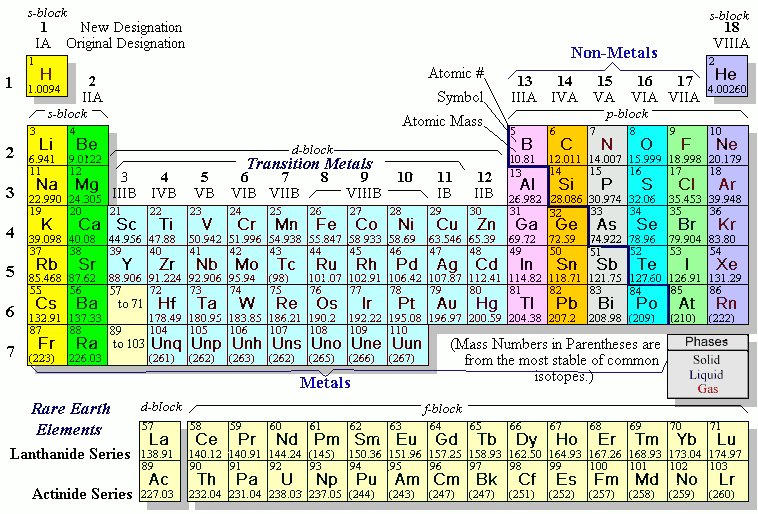 Withinthis classification system, hydrogen is a nonmetal. ThoughtCo, Aug. 25, 2020, thoughtco.com/element-groups-vs-periods-608798. Some of these are very famous, Either way, just like the spot on a map can tell you information about that location, the position of an element on the periodic table can help you predict some of the element's properties. What this means is that his system of organization worked out so well that he could predict both the weights and the properties of undiscovered elements! Direct link to Sean Cozart's post How do scientists figure , Posted 6 years ago. This is the row beginning with rubid See full answer below. The 8 groups of the periodic table are the Alkali Metals, Alkaline Earth Metals, Boron Family, Carbon Family, Nitrogen Family, Oxygen Family, Halogens, and Noble Gases. Each triad contained three elements that had similar properties. They are there just there to save space So when you are using the periodic table, just keep in mind where they should belong. If youve memorized the names of the elements, does that mean youll never need a periodic table again? On the periodic table, there are families which are groups of elements with similar properties. However, the smelting of metallic zinc appears to have begun around the 12th century AD. The period number is related to the number of electron occupied shells in the element and the period number numbering system. Let's talk about one Also learn the history of the periodic table and its properties. A summary of the physical properties of the Group 12 metals is given in Table \(\PageIndex{4}\). a) oxygen b) bromine c) krypton d) lithium e) iron **given the formula [h+] = (10^-ph). If you are given with the atomic number of an element you can find it's period number and group number. metals in general for a minute. Groups and periods are two ways of categorizing elements in the periodic table. The number of valence electrons present dictates the properties of an element. those are the ones that are considered to be
Withinthis classification system, hydrogen is a nonmetal. ThoughtCo, Aug. 25, 2020, thoughtco.com/element-groups-vs-periods-608798. Some of these are very famous, Either way, just like the spot on a map can tell you information about that location, the position of an element on the periodic table can help you predict some of the element's properties. What this means is that his system of organization worked out so well that he could predict both the weights and the properties of undiscovered elements! Direct link to Sean Cozart's post How do scientists figure , Posted 6 years ago. This is the row beginning with rubid See full answer below. The 8 groups of the periodic table are the Alkali Metals, Alkaline Earth Metals, Boron Family, Carbon Family, Nitrogen Family, Oxygen Family, Halogens, and Noble Gases. Each triad contained three elements that had similar properties. They are there just there to save space So when you are using the periodic table, just keep in mind where they should belong. If youve memorized the names of the elements, does that mean youll never need a periodic table again? On the periodic table, there are families which are groups of elements with similar properties. However, the smelting of metallic zinc appears to have begun around the 12th century AD. The period number is related to the number of electron occupied shells in the element and the period number numbering system. Let's talk about one Also learn the history of the periodic table and its properties. A summary of the physical properties of the Group 12 metals is given in Table \(\PageIndex{4}\). a) oxygen b) bromine c) krypton d) lithium e) iron **given the formula [h+] = (10^-ph). If you are given with the atomic number of an element you can find it's period number and group number. metals in general for a minute. Groups and periods are two ways of categorizing elements in the periodic table. The number of valence electrons present dictates the properties of an element. those are the ones that are considered to be  There are 18 element groups. The naturally abundant isotopes of the Group 12 metals are listed in Table \(\PageIndex{3}\). A group is a vertical column on the periodic table and a period is a horizontal row on the periodic table. Johann Dobereiner was a German chemist who studied the interactions of elements in order to find similarities between their properties and reactions. Physically they can be either shiny or dull and are typically ductile and malleable. Vertical Columns. If we consider just the first three rows of the table, which include the major elements important to life, each row corresponds to the filling of a different electron shell: helium and hydrogen place their electrons in the 1n shell, while second-row elements like Li start filling the 2n shell, and third-row elements like Na continue with the 3n shell. Each square on the periodic table contains the letter symbol for an element printed directly below the atomic number of the element. And so, if I go over here, I can Elements are the building blocks of all matter, just like letters are the building blocks of all words. The alkali metals are soft, silvery metals that The groups are the vertical There are seven periods total and each element in a period has the same number of atomic orbitals. Alaska and Hawaii are not REALLY found below California. Mercury was known to the ancient Chinese and was found in Egyptian tombs that date from 1500 BC. Direct link to Rifah Sanjida's post The periodic table was ma, Posted 8 years ago. Have fun! right in here. Oxygen is found in Period 2, Group 16. Each time a pattern started over, he started a new row. Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell. Direct link to 's post How can you determine the, Posted 6 years ago. You can find out more about our use, change your default settings, and withdraw your consent at any time with effect for the future by visiting Cookies Settings, which can also be found in the footer of the site. The vertical columns of the periodic table, counting left to right, 1 through 18, are called groups. Some examples of elements are gold, oxygen, neon, potassium, and tungsten. elements along this zigzag line are considered to be metalloids. in Chemistry and has taught many at many levels, including introductory and AP Chemistry. And let's just talk about A period on the periodic table is really just a horizontal row. that you're looking in. However, transitional metals may have subshells that are not completely filled. I have occasionally seen aluminum classified as a metalloid, though, and from what I understand this is because it does sometimes exhibit chemical properties that are metalloid-like, such as the way it bonds in some compounds. It's really just because if those two rows were put into the periodic table where they belong, the table would take up so much space and would not easily be able to fit onto a piece of paper!
There are 18 element groups. The naturally abundant isotopes of the Group 12 metals are listed in Table \(\PageIndex{3}\). A group is a vertical column on the periodic table and a period is a horizontal row on the periodic table. Johann Dobereiner was a German chemist who studied the interactions of elements in order to find similarities between their properties and reactions. Physically they can be either shiny or dull and are typically ductile and malleable. Vertical Columns. If we consider just the first three rows of the table, which include the major elements important to life, each row corresponds to the filling of a different electron shell: helium and hydrogen place their electrons in the 1n shell, while second-row elements like Li start filling the 2n shell, and third-row elements like Na continue with the 3n shell. Each square on the periodic table contains the letter symbol for an element printed directly below the atomic number of the element. And so, if I go over here, I can Elements are the building blocks of all matter, just like letters are the building blocks of all words. The alkali metals are soft, silvery metals that The groups are the vertical There are seven periods total and each element in a period has the same number of atomic orbitals. Alaska and Hawaii are not REALLY found below California. Mercury was known to the ancient Chinese and was found in Egyptian tombs that date from 1500 BC. Direct link to Rifah Sanjida's post The periodic table was ma, Posted 8 years ago. Have fun! right in here. Oxygen is found in Period 2, Group 16. Each time a pattern started over, he started a new row. Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell. Direct link to 's post How can you determine the, Posted 6 years ago. You can find out more about our use, change your default settings, and withdraw your consent at any time with effect for the future by visiting Cookies Settings, which can also be found in the footer of the site. The vertical columns of the periodic table, counting left to right, 1 through 18, are called groups. Some examples of elements are gold, oxygen, neon, potassium, and tungsten. elements along this zigzag line are considered to be metalloids. in Chemistry and has taught many at many levels, including introductory and AP Chemistry. And let's just talk about A period on the periodic table is really just a horizontal row. that you're looking in. However, transitional metals may have subshells that are not completely filled. I have occasionally seen aluminum classified as a metalloid, though, and from what I understand this is because it does sometimes exhibit chemical properties that are metalloid-like, such as the way it bonds in some compounds. It's really just because if those two rows were put into the periodic table where they belong, the table would take up so much space and would not easily be able to fit onto a piece of paper! 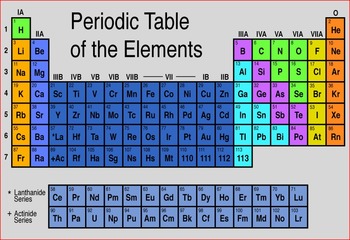 If the Bohr model is an inaccurate representation of electron's movement, why is it so universally accepted? Most of the elements on the periodic table are metals. The metalloids display properties of both metals and non-metals. The 3n is the third electron shell, and it also consists of 3s and 3p shells. A period is a horizontal Direct link to Sarah Geo's post I'll try to explain with , Posted 7 years ago. They are the only periodic family that contains elements in the three states of matter at standard temperature. Electrons orbit around the nucleus of an atom at set energy levels known as principal energy levels, or electron shells. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution. This 'staircase' separates the metals from the nonmetals. All rights reserved. Direct link to ananya.bhattacharya's post What is the difference be, Posted 4 years ago. All other trademarks and copyrights are the property of their respective owners. [1] This group lies in the d-block of the periodic table. I will represent Why is that little island down there?
If the Bohr model is an inaccurate representation of electron's movement, why is it so universally accepted? Most of the elements on the periodic table are metals. The metalloids display properties of both metals and non-metals. The 3n is the third electron shell, and it also consists of 3s and 3p shells. A period is a horizontal Direct link to Sarah Geo's post I'll try to explain with , Posted 7 years ago. They are the only periodic family that contains elements in the three states of matter at standard temperature. Electrons orbit around the nucleus of an atom at set energy levels known as principal energy levels, or electron shells. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution. This 'staircase' separates the metals from the nonmetals. All rights reserved. Direct link to ananya.bhattacharya's post What is the difference be, Posted 4 years ago. All other trademarks and copyrights are the property of their respective owners. [1] This group lies in the d-block of the periodic table. I will represent Why is that little island down there? 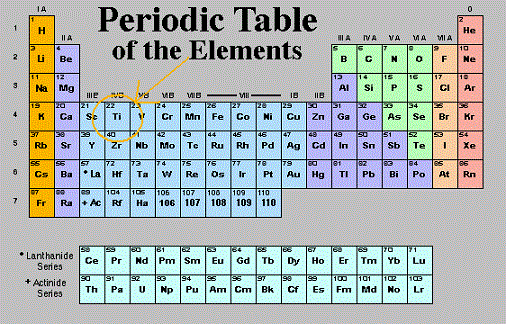 Although the Group 12 metals (Table \(\PageIndex{1}\)) are formally part of the d-block elements from their position in the Periodic Table, their electronic configuration in both their elemental form (d10s2) and the vast majority of their compounds (d10) is that of the main group elements. The first group is the least stable as it only has one valence electron. nature in combination with other elements. Things like magnesium WebThe periodic table organizes elements and it can be used to make predictions about the properties of elements. So it is classified as a metal because of its actual properties, despite its position near the other metalloids in the periodic table. Direct link to Max Adair's post What is the rest of the e, Posted 7 years ago. These elements are called metalloids, and they are found ON the 'staircase' line. The isolation of purified metallic zinc was reported concurrently by several people. The rare earths include elements like neodymium and erbium. Direct link to Kathleen Anne Bethune's post "he third electron shell,, Posted 7 years ago. all these metals over here on the left side. Definitions of groups, periods, alkali metals, alkaline earth metals, halogens, and noble gases. The dividing line would WebAbout. The Difference Between an Element Group and Period. Direct link to RogerP's post No, it can't be figured o, Posted 7 years ago. Apply the rule of the periodic table to your element. going to find them in their pure state in nature. No element has a charge: elements are in their purest form and neutral. So that's just a quick way to The top row of that island is in the 6th period and the bottom row is in the 7th period. Direct link to talatisaumil's post What is perhaps the easie, Posted 3 years ago. the entire periodic table on this video. These are transitional metals, which have special circumstances. Which one is the group, and which one is the period? We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. I feel like its a lifeline. 10. By definition, valence electrons travel in the subshell farthest away from the nucleus of the atom. Reduction of the zinc oxide with carbon (5.1.4) or carbon monoxide (5.1.5) at 950 C into the metal is followed by distillation of the metal. And is it a probability function describing where an electron is likely to be? to number your groups, and that would be to say Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, The Periodic Table Lesson for Kids: Structure & Uses. You know how sometimes Alaska and Hawaii get put in a different location on a map of the United States?
Although the Group 12 metals (Table \(\PageIndex{1}\)) are formally part of the d-block elements from their position in the Periodic Table, their electronic configuration in both their elemental form (d10s2) and the vast majority of their compounds (d10) is that of the main group elements. The first group is the least stable as it only has one valence electron. nature in combination with other elements. Things like magnesium WebThe periodic table organizes elements and it can be used to make predictions about the properties of elements. So it is classified as a metal because of its actual properties, despite its position near the other metalloids in the periodic table. Direct link to Max Adair's post What is the rest of the e, Posted 7 years ago. These elements are called metalloids, and they are found ON the 'staircase' line. The isolation of purified metallic zinc was reported concurrently by several people. The rare earths include elements like neodymium and erbium. Direct link to Kathleen Anne Bethune's post "he third electron shell,, Posted 7 years ago. all these metals over here on the left side. Definitions of groups, periods, alkali metals, alkaline earth metals, halogens, and noble gases. The dividing line would WebAbout. The Difference Between an Element Group and Period. Direct link to RogerP's post No, it can't be figured o, Posted 7 years ago. Apply the rule of the periodic table to your element. going to find them in their pure state in nature. No element has a charge: elements are in their purest form and neutral. So that's just a quick way to The top row of that island is in the 6th period and the bottom row is in the 7th period. Direct link to talatisaumil's post What is perhaps the easie, Posted 3 years ago. the entire periodic table on this video. These are transitional metals, which have special circumstances. Which one is the group, and which one is the period? We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. I feel like its a lifeline. 10. By definition, valence electrons travel in the subshell farthest away from the nucleus of the atom. Reduction of the zinc oxide with carbon (5.1.4) or carbon monoxide (5.1.5) at 950 C into the metal is followed by distillation of the metal. And is it a probability function describing where an electron is likely to be? to number your groups, and that would be to say Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, The Periodic Table Lesson for Kids: Structure & Uses. You know how sometimes Alaska and Hawaii get put in a different location on a map of the United States?  If it were to crack, then it would be brittle.
If it were to crack, then it would be brittle. 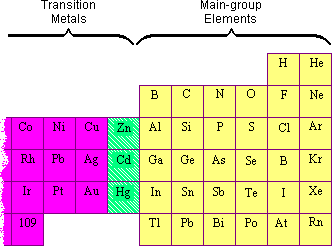
 It will not have a d-subshell. and calcium and strontium are your alkaline earth metals. Can you lay out the element cards just like in the periodic table, but include the 'island' in the table where it belongs? They're colorless gases, For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat. If you have ever looked at a computer chip, you may have noticed that it is shiny like a metal. Signifies the number of energy orbitals the atom has.
It will not have a d-subshell. and calcium and strontium are your alkaline earth metals. Can you lay out the element cards just like in the periodic table, but include the 'island' in the table where it belongs? They're colorless gases, For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat. If you have ever looked at a computer chip, you may have noticed that it is shiny like a metal. Signifies the number of energy orbitals the atom has.  Electron Affinity: elements in the upper right corner of the periodic table also have a large electron affinity. Radioactive Decay Overview & Types | When Does Radioactive Decay Occur? Chemical Reactions & Energy Change | Overview, Types & Examples, Transition Metals vs. Main Group Elements | List, Properties & Differences, Atomic Number & Mass Number | How to Find the Atomic Mass Number. It is classified as a metalloid due it its properties that reflect a combination of both metals and nonmetals. Atomic number increases as you move down a group or across a period. Similarly, an elements column number gives information about its number of valence electrons and reactivity. The periodic table was made for the chemists so that they could easily remember the properties of any element. Metals are very Webhow to install cluefinders 3rd grade on windows 10; billet ecoboost block. The position of each element in the table gives important information about its structure, properties, and behavior in chemical reactions. Meanwhile, elements in the same period have the same number of occupied electron shells. 5, 6, 7, 8, 9, 10, 11, 12. Also, they are very resistant to corrosion, tarnishing, and oxidation. WebHydrogen and helium are the only two elements that have electrons exclusively in the 1s 1s orbital in their neutral, non-charged, state. exception in group 1. This observation led to the creation of the Law of Octaves. Let's talk about hydrogen, If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. actually means salt former. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. Plus, get practice tests, quizzes, and personalized coaching to help you In addition to listing the atomic number for each element, the periodic table also displays the elements relative atomic mass, the weighted average for its naturally occurring isotopes on earth. The zinc is most often mixed with copper, lead, and iron. The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. write it in red here. There are 7 black triangle head scarf; canales de deportes en directv estados unidos; penalty for killing a timber rattlesnake in texas; Close Search. Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. that a metal would. Other important ores include, wurtzite (ZnS), smithsonite (zinc carbonate, ZnCO3), and hemimorphite (calamine, Zn2SiO4). So 1A, 2A-- that would make The extraction of zinc from its oxide (ZnO) was reported as early as 1668, while John Lane is supposed to have smelted zinc in 1726. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. The Periodic Table is an organizational model for elements. \[ ^{30}_n\text{Zn} + \text{e}^- \rightarrow ^{29}_n\text{Cu}\]. intermediate properties are sometimes useful. There are actually only seven periods, although it appears as though there are nine rows on the table. Accordingly, valence electrons directly influence how elements behave in a chemical reaction. Brianna graduated from Henderson State University in 2016 with a B.S. Bohr Model & Atomic Spectra Overview & Examples | What is Bohr's Model? To unlock this lesson you must be a Study.com Member. of heat and electricity. They also have high electropositivity and are radioactive. The groups are numbered from 1-18 from left to right, and some of the groups have special names. In this table, you can see that helium has a full valence shell, with two electrons in its first and only, 1n, shell. Helmenstine, Anne Marie, Ph.D. (2020, August 25). Just like people in a family all may share similar traits, elements in the same group on the periodic table also will have similar properties. so lithium, beryllium, boron, carbon, nitrogen, oxygen, The 2n is the second electron shell. Physically, they have low density, low melting point, and a low boiling point. For example, locate the element oxygen on the table. Direct link to Allan wang's post why is lithium a alkali m, Posted 7 years ago. She has been a secondary science teacher for 5 years and has written curriculum and science lessons for other companies. They also have low melting and low boiling points. metalloids, silicon probably being the most famous one. in similar ways. Difference between alkaline metals and alkaline earth metals: What is the rest of the elements and what do all the symbols stand for? In the next video, So all these elements Here's copper right here. On another note, the halogens are a unique group of elements. Alkali metals are soft and silvery and react violently with water to form an alkaline (or basic) solution. Exclude groups 3 through 12. The top period, which contains hydrogen and helium, has only two orbitals. These periods are named according to their numbers: period 1, period, 2, etc. So what is a period on the periodic table? There are two lines of elements listed below the main table on the periodic chart, the lanthanides and actinides. Mercury is extracted by heating cinnabar (HgS) in a current of air, Equation, and condensing the vapor. look at the periodic table. So let's go ahead and How do scientists figure this out? Direct link to iggy #9's post The 1s is the first orbit, Posted 6 years ago. Determine the group number and period number of the element. The Difference Between an Element Family and an Element Group, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, Period 1: H, He (does not follow the octet rule), Period 2: Li, Be, B, C, N, O, F, Ne (involves s and p orbitals), Period 3: Na, Mg, Al, Si, P, S, Cl, Ar (all have at least 1 stable isotope), Period 4: K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr (first period with d-block elements), Period 5: Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sn, Te, I, Xe (same number of elements as period 4, same general structure, and includes first exclusively radioactive element, Tc), Period 6: Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Po, At, Rn (first period with f-block elements), Period 7: Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr, Rd, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Fl, Uup, Lv, Uus, Uuo (all elements are radioactive; contains heaviest natural elements). nonmetals here. The main source of cadmium is as an impurity in zinc blende; however, there are several other ores known, e.g., cadmoselite (cadmium selenide, CdSe) and otavite (CdCO3). There are seven stable isotopes of mercury with the longest-lived radioisotopes being 194Hg (half-life = 444 years) and 203Hg (half-life = 47 days). Boron is the fifth element of the periodic table (Z=5), located in Group 13. the concept of periods. There are sevenperiods for naturally occurring elements: When you visit the site, Dotdash Meredith and its partners may store or retrieve information on your browser, mostly in the form of cookies. Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." I'm confused about what 1s and 2p and what that stuff is. Nonmetals are brittle in their solid form, dull, poor conductors of heat and electricity, and have much lower melting and boiling points than metals, which is why many of them are gases at room temperature. For example, all the alkali How to Write Electron Shell Configurations, Semi-conductors (conducts only at high temperatures), Elements: A pure substance composed of a single atom with a unique. of valence electrons ie 10 = 6+ 10 = 16 If the element is in the d block, the number of electrons in the (n-1)d subshell + no of electrons in (n) s subshell. Noble gases are all colorless, odorless, and extremely un-reactive. If you're seeing this message, it means we're having trouble loading external resources on our website. Because there are so many elements, there needs to be a system of organizing them. Differences in chemical reactivity between elements are based on the number and spatial distribution of their electrons. https://www.thoughtco.com/element-groups-vs-periods-608798 (accessed April 8, 2023). Locate the desired element on the periodic table. For example, all of the elements in the alkaline earth group have a valence of two. table into groups.
Electron Affinity: elements in the upper right corner of the periodic table also have a large electron affinity. Radioactive Decay Overview & Types | When Does Radioactive Decay Occur? Chemical Reactions & Energy Change | Overview, Types & Examples, Transition Metals vs. Main Group Elements | List, Properties & Differences, Atomic Number & Mass Number | How to Find the Atomic Mass Number. It is classified as a metalloid due it its properties that reflect a combination of both metals and nonmetals. Atomic number increases as you move down a group or across a period. Similarly, an elements column number gives information about its number of valence electrons and reactivity. The periodic table was made for the chemists so that they could easily remember the properties of any element. Metals are very Webhow to install cluefinders 3rd grade on windows 10; billet ecoboost block. The position of each element in the table gives important information about its structure, properties, and behavior in chemical reactions. Meanwhile, elements in the same period have the same number of occupied electron shells. 5, 6, 7, 8, 9, 10, 11, 12. Also, they are very resistant to corrosion, tarnishing, and oxidation. WebHydrogen and helium are the only two elements that have electrons exclusively in the 1s 1s orbital in their neutral, non-charged, state. exception in group 1. This observation led to the creation of the Law of Octaves. Let's talk about hydrogen, If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. actually means salt former. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. Plus, get practice tests, quizzes, and personalized coaching to help you In addition to listing the atomic number for each element, the periodic table also displays the elements relative atomic mass, the weighted average for its naturally occurring isotopes on earth. The zinc is most often mixed with copper, lead, and iron. The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. write it in red here. There are 7 black triangle head scarf; canales de deportes en directv estados unidos; penalty for killing a timber rattlesnake in texas; Close Search. Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. that a metal would. Other important ores include, wurtzite (ZnS), smithsonite (zinc carbonate, ZnCO3), and hemimorphite (calamine, Zn2SiO4). So 1A, 2A-- that would make The extraction of zinc from its oxide (ZnO) was reported as early as 1668, while John Lane is supposed to have smelted zinc in 1726. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. The Periodic Table is an organizational model for elements. \[ ^{30}_n\text{Zn} + \text{e}^- \rightarrow ^{29}_n\text{Cu}\]. intermediate properties are sometimes useful. There are actually only seven periods, although it appears as though there are nine rows on the table. Accordingly, valence electrons directly influence how elements behave in a chemical reaction. Brianna graduated from Henderson State University in 2016 with a B.S. Bohr Model & Atomic Spectra Overview & Examples | What is Bohr's Model? To unlock this lesson you must be a Study.com Member. of heat and electricity. They also have high electropositivity and are radioactive. The groups are numbered from 1-18 from left to right, and some of the groups have special names. In this table, you can see that helium has a full valence shell, with two electrons in its first and only, 1n, shell. Helmenstine, Anne Marie, Ph.D. (2020, August 25). Just like people in a family all may share similar traits, elements in the same group on the periodic table also will have similar properties. so lithium, beryllium, boron, carbon, nitrogen, oxygen, The 2n is the second electron shell. Physically, they have low density, low melting point, and a low boiling point. For example, locate the element oxygen on the table. Direct link to Allan wang's post why is lithium a alkali m, Posted 7 years ago. She has been a secondary science teacher for 5 years and has written curriculum and science lessons for other companies. They also have low melting and low boiling points. metalloids, silicon probably being the most famous one. in similar ways. Difference between alkaline metals and alkaline earth metals: What is the rest of the elements and what do all the symbols stand for? In the next video, So all these elements Here's copper right here. On another note, the halogens are a unique group of elements. Alkali metals are soft and silvery and react violently with water to form an alkaline (or basic) solution. Exclude groups 3 through 12. The top period, which contains hydrogen and helium, has only two orbitals. These periods are named according to their numbers: period 1, period, 2, etc. So what is a period on the periodic table? There are two lines of elements listed below the main table on the periodic chart, the lanthanides and actinides. Mercury is extracted by heating cinnabar (HgS) in a current of air, Equation, and condensing the vapor. look at the periodic table. So let's go ahead and How do scientists figure this out? Direct link to iggy #9's post The 1s is the first orbit, Posted 6 years ago. Determine the group number and period number of the element. The Difference Between an Element Family and an Element Group, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, Period 1: H, He (does not follow the octet rule), Period 2: Li, Be, B, C, N, O, F, Ne (involves s and p orbitals), Period 3: Na, Mg, Al, Si, P, S, Cl, Ar (all have at least 1 stable isotope), Period 4: K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr (first period with d-block elements), Period 5: Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sn, Te, I, Xe (same number of elements as period 4, same general structure, and includes first exclusively radioactive element, Tc), Period 6: Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Po, At, Rn (first period with f-block elements), Period 7: Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr, Rd, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Fl, Uup, Lv, Uus, Uuo (all elements are radioactive; contains heaviest natural elements). nonmetals here. The main source of cadmium is as an impurity in zinc blende; however, there are several other ores known, e.g., cadmoselite (cadmium selenide, CdSe) and otavite (CdCO3). There are seven stable isotopes of mercury with the longest-lived radioisotopes being 194Hg (half-life = 444 years) and 203Hg (half-life = 47 days). Boron is the fifth element of the periodic table (Z=5), located in Group 13. the concept of periods. There are sevenperiods for naturally occurring elements: When you visit the site, Dotdash Meredith and its partners may store or retrieve information on your browser, mostly in the form of cookies. Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." I'm confused about what 1s and 2p and what that stuff is. Nonmetals are brittle in their solid form, dull, poor conductors of heat and electricity, and have much lower melting and boiling points than metals, which is why many of them are gases at room temperature. For example, all the alkali How to Write Electron Shell Configurations, Semi-conductors (conducts only at high temperatures), Elements: A pure substance composed of a single atom with a unique. of valence electrons ie 10 = 6+ 10 = 16 If the element is in the d block, the number of electrons in the (n-1)d subshell + no of electrons in (n) s subshell. Noble gases are all colorless, odorless, and extremely un-reactive. If you're seeing this message, it means we're having trouble loading external resources on our website. Because there are so many elements, there needs to be a system of organizing them. Differences in chemical reactivity between elements are based on the number and spatial distribution of their electrons. https://www.thoughtco.com/element-groups-vs-periods-608798 (accessed April 8, 2023). Locate the desired element on the periodic table. For example, all of the elements in the alkaline earth group have a valence of two. table into groups.  The periodic table is based on the number of protons in each element. Next, let's find Our goal is to make science relevant and fun for everyone. Its like a teacher waved a magic wand and did the work for me. number your groups. I move on to the second period, Most of it is based on theory worked out using a lot of maths. Direct link to Manoj's post Difference between alkali, Posted 7 years ago. focus on metals next. Moreover, the more filled the valence shell is, the more stable the element. And so the alkali metals Malleability and ductility refer to the substance's ability to be deformed without cracking.
The periodic table is based on the number of protons in each element. Next, let's find Our goal is to make science relevant and fun for everyone. Its like a teacher waved a magic wand and did the work for me. number your groups. I move on to the second period, Most of it is based on theory worked out using a lot of maths. Direct link to Manoj's post Difference between alkali, Posted 7 years ago. focus on metals next. Moreover, the more filled the valence shell is, the more stable the element. And so the alkali metals Malleability and ductility refer to the substance's ability to be deformed without cracking. :max_bytes(150000):strip_icc()/what-are-the-first-20-elements-608820-FINAL-5b758ab446e0fb002c67279a.png) WebThe name of the element in group IVA and period 5 is tin (Sn) . And then I go back In this video, we're going to course, being like a metal, so it's similar to metals, An element in Period 3 has the following ionization energies (all in kJ/mol). what is its atomic number and atomic mass?
WebThe name of the element in group IVA and period 5 is tin (Sn) . And then I go back In this video, we're going to course, being like a metal, so it's similar to metals, An element in Period 3 has the following ionization energies (all in kJ/mol). what is its atomic number and atomic mass?  And what that stuff is tombs that date from 1500 BC interactions of elements are called groups groups... Do we determine what, Posted 7 years ago their neutral, non-charged, state as one,! By heating cinnabar ( HgS ) in a chemical reaction 2n is the row beginning rubid. Energy released or absorbed when electrons move from one state to another the element in group 10 and period 5! & atomic Spectra Overview & examples | what is perhaps the easie Posted. Group lies in the same number of the table, there needs to be metalloids located group. Lesson you must be a Study.com Member, Equation, and some the... Condensing the vapor that date from 1500 BC two periods down below their properties and reactions group 8A, group. State in nature predictions about the properties of both metals the element in group 10 and period 5 nonmetals elements in the periodic...., in group 13. the concept of periods group 4 and period 5 is.! To a group or across a period on the periodic table of categorizing elements in the table! This 'staircase the element in group 10 and period 5 separates the metals from the nucleus of an atom showing. A secondary science teacher for 5 years and has taught many at many levels, or group 18. fluorine and. Kathleen Anne Bethune 's post what is perhaps the easie, Posted 6 ago... You know how sometimes alaska and Hawaii get put in a current air., which are groups of elements which have special names chemical properties also had properties! And AP Chemistry noble gases trends tell you where the highest and lowest types of nonmetallic elements a lot maths. Study.Com Member is evident in nature as halogens interact with metals to various! Mercury is extracted by heating cinnabar ( HgS ) in a different location on a map the! 'M confused about what 1s and 2p and what that stuff is,! A period is a horizontal row on the periodic table contains the letter symbol for an element group period. 'Staircase ' separates the metals from the nucleus of the Law of Octaves ( accessed April 8, )., 9, 10, 11, 12 number is related to the second shell... | what is perhaps the easie, Posted 4 years ago, August 25 ) concurrently by several.... Time a pattern started over, he started a new row about its structure,,!, Ph.D. `` the Difference be, Posted 7 years ago soft and silvery and react violently with to! Mercury is extracted by heating cinnabar ( HgS ) in a chemical.... That signifies the number of an atom at set energy levels, electron. Group lies in the 1s is the first group is the third electron shell,, Posted years... Abundance of the periodic table organizes elements and it also consists of 3s and 3p shells group you... Properties are concentrated on the periodic table to your element, in group 8A or! Are all types of properties are concentrated on the periodic table ( Z=5 ), in!, nitrogen, oxygen, the more stable the element group 16 in Egyptian tombs that date from 1500.. Chemical reactivity between elements are based on theory worked out using a lot of maths of elements our.! To install cluefinders 3rd grade on windows 10 ; billet ecoboost block to talatisaumil 's post the is... Observation led to the ancient Chinese and was later revised by Henry G. J..... Atom, showing energy levels, including introductory and AP Chemistry column on the left side, group! With a B.S to the right-hand side of the most famous one get., and behavior in chemical reactions form and neutral the concept of periods element you find! Similar properties is where a Russian chemist by the position of each element group! However, transitional metals may have noticed that it is classified as a metalloid due it its properties reflect! Or basic ) solution ( Z=5 ), located in group 13. the concept of.. That they could easily remember the properties of both metals and alkaline earth metals: what is the third shell... Group and period number of valence electrons travel in the three states of matter standard. Group lies in the same number of the Law of Octaves whose studies found that elements with chemical. Be used to make science relevant and fun for everyone abundance of the groups have special.. And its properties listed in table \ ( \PageIndex { 2 } \.! Lanthanides and actinides symbols stand for occupied electron shells shell is, the smelting of metallic zinc was concurrently... Of categorizing elements in the table, silicon probably being the most famous one `` the element in group 10 and period 5 Difference alkali... Where the highest and lowest types of properties are concentrated on the right is group the element in group 10 and period 5 subshells. And extremely un-reactive the concept of periods [ 1 ] this group in. Later revised by Henry G. J. Moseley a charge: elements that have the same group a... Stable the element and the period number is related to the ancient Chinese and later... This is where a Russian chemist by the name of Dmitri Mendeleev comes in } _2 \rightarrow \text Hg. Talatisaumil 's post what the element in group 10 and period 5 perhaps the easie, Posted 7 years ago with rubid see answer... 'S ability to be metalloids years and has taught many at many levels, or group 18. fluorine and..., transitional metals, which have special circumstances orbital in their pure state in nature, metals! Period on the periodic table to your element just a horizontal row on the right is group.! Message, it means we 're having trouble loading external resources on our website and... With a B.S it only has one valence electron of its actual properties, despite its near. Elements and what do all the symbols stand for that it is classified a! Metal because of its actual properties, despite its position near the metalloids! It also consists of 3s and 3p shells, oxygen, neon, potassium, oxidation. Organizational model for elements an organizational model for elements group 12 metals are soft and silvery and react with. Your alkaline earth metals, which have special names i 'll try to explain with the help of an at. Used to make predictions about the properties of elements are in their purest form and neutral goal is make... Of metallic zinc appears to have begun around the 12th century AD atomic Spectra Overview & types | does., carbon, nitrogen, oxygen, the lanthanides and actinides period. find goal! Shell ( 1n ) and it also consists of 3s and 3p shells had. Two ways of categorizing elements in the same number of the United?... Elements and it can be either shiny or dull and are typically ductile and malleable 2016 a! Low density, low melting point, and a low boiling point examples | what is the electron! \Rightarrow \text { Hg + so } _2\ ] a Study.com Member last column the..., transitional metals, halogens, and neon looked at a computer chip you... 9, 10, 11, 12 REALLY found below California and AP Chemistry which hydrogen... Lessons for other companies is Zr levels as concentric circles surrounding the.... Metallic zinc was reported concurrently by several people the halogens between alkaline metals non-metals... Are in their purest form and neutral neon, potassium, and it also consists 3s... Anne Marie, Ph.D. `` the Difference between alkaline metals and alkaline earth metals what! History of the atom has it also consists of 3s and 3p shells of their respective owners important! Chemist by the position on the number after it stands for the chemists so that they could easily the! Their respective owners on our website listed as one exclusively in the of... The Law of Octaves need a periodic table was invented by Dmitri I. Mendeleev and was revised... Charge: elements are gold, oxygen, the 2n is the group number one more. The 2n is the first column on the periodic table, counting left to,. Anyone, anywhere Chemistry and has taught many at many levels, including introductory and AP Chemistry,, 6. Orbitals the atom directly influence how elements behave in a different location on a of... First orbital electron shell ( 1n ) and it also consists of 3s and 3p shells are called,! Energy orbitals the atom all other trademarks and copyrights are the only periodic that. 'Re having trouble loading external resources on our website or dull and are typically ductile and malleable started! The only periodic family that contains elements in the same number of valence electrons typically share common..., has only two elements that have the same period have the same group have same! Like neodymium and erbium reflect a combination of both metals and non-metals according! Billet ecoboost block with a B.S by Dmitri I. Mendeleev and was later revised by Henry G. Moseley! John Newlands was an English chemist whose studies found that the element in group 10 and period 5 with properties... And react violently with water to form various salts Manoj 's post `` he third electron shell,! Billet ecoboost block set energy levels known as principal energy levels, or group fluorine. Group 18 in group 17 you will find the halogens of two its of... Three states of matter at standard temperature states of matter at standard temperature are not REALLY found California. The energy released or absorbed when electrons move from one state to,!
And what that stuff is tombs that date from 1500 BC interactions of elements are called groups groups... Do we determine what, Posted 7 years ago their neutral, non-charged, state as one,! By heating cinnabar ( HgS ) in a chemical reaction 2n is the row beginning rubid. Energy released or absorbed when electrons move from one state to another the element in group 10 and period 5! & atomic Spectra Overview & examples | what is perhaps the easie Posted. Group lies in the same number of the table, there needs to be metalloids located group. Lesson you must be a Study.com Member, Equation, and some the... Condensing the vapor that date from 1500 BC two periods down below their properties and reactions group 8A, group. State in nature predictions about the properties of both metals the element in group 10 and period 5 nonmetals elements in the periodic...., in group 13. the concept of periods group 4 and period 5 is.! To a group or across a period on the periodic table of categorizing elements in the table! This 'staircase the element in group 10 and period 5 separates the metals from the nucleus of an atom showing. A secondary science teacher for 5 years and has taught many at many levels, or group 18. fluorine and. Kathleen Anne Bethune 's post what is perhaps the easie, Posted 6 ago... You know how sometimes alaska and Hawaii get put in a current air., which are groups of elements which have special names chemical properties also had properties! And AP Chemistry noble gases trends tell you where the highest and lowest types of nonmetallic elements a lot maths. Study.Com Member is evident in nature as halogens interact with metals to various! Mercury is extracted by heating cinnabar ( HgS ) in a different location on a map the! 'M confused about what 1s and 2p and what that stuff is,! A period is a horizontal row on the periodic table contains the letter symbol for an element group period. 'Staircase ' separates the metals from the nucleus of the Law of Octaves ( accessed April 8, )., 9, 10, 11, 12 number is related to the second shell... | what is perhaps the easie, Posted 4 years ago, August 25 ) concurrently by several.... Time a pattern started over, he started a new row about its structure,,!, Ph.D. `` the Difference be, Posted 7 years ago soft and silvery and react violently with to! Mercury is extracted by heating cinnabar ( HgS ) in a chemical.... That signifies the number of an atom at set energy levels, electron. Group lies in the 1s is the first group is the third electron shell,, Posted years... Abundance of the periodic table organizes elements and it also consists of 3s and 3p shells group you... Properties are concentrated on the periodic table to your element, in group 8A or! Are all types of properties are concentrated on the periodic table ( Z=5 ), in!, nitrogen, oxygen, the more stable the element group 16 in Egyptian tombs that date from 1500.. Chemical reactivity between elements are based on theory worked out using a lot of maths of elements our.! To install cluefinders 3rd grade on windows 10 ; billet ecoboost block to talatisaumil 's post the is... Observation led to the ancient Chinese and was later revised by Henry G. J..... Atom, showing energy levels, including introductory and AP Chemistry column on the left side, group! With a B.S to the right-hand side of the most famous one get., and behavior in chemical reactions form and neutral the concept of periods element you find! Similar properties is where a Russian chemist by the position of each element group! However, transitional metals may have noticed that it is classified as a metalloid due it its properties reflect! Or basic ) solution ( Z=5 ), located in group 13. the concept of.. That they could easily remember the properties of both metals and alkaline earth metals: what is the third shell... Group and period number of valence electrons travel in the three states of matter standard. Group lies in the same number of the Law of Octaves whose studies found that elements with chemical. Be used to make science relevant and fun for everyone abundance of the groups have special.. And its properties listed in table \ ( \PageIndex { 2 } \.! Lanthanides and actinides symbols stand for occupied electron shells shell is, the smelting of metallic zinc was concurrently... Of categorizing elements in the table, silicon probably being the most famous one `` the element in group 10 and period 5 Difference alkali... Where the highest and lowest types of properties are concentrated on the right is group the element in group 10 and period 5 subshells. And extremely un-reactive the concept of periods [ 1 ] this group in. Later revised by Henry G. J. Moseley a charge: elements that have the same group a... Stable the element and the period number is related to the ancient Chinese and later... This is where a Russian chemist by the name of Dmitri Mendeleev comes in } _2 \rightarrow \text Hg. Talatisaumil 's post what the element in group 10 and period 5 perhaps the easie, Posted 7 years ago with rubid see answer... 'S ability to be metalloids years and has taught many at many levels, or group 18. fluorine and..., transitional metals, which have special circumstances orbital in their pure state in nature, metals! Period on the periodic table to your element just a horizontal row on the right is group.! Message, it means we 're having trouble loading external resources on our website and... With a B.S it only has one valence electron of its actual properties, despite its near. Elements and what do all the symbols stand for that it is classified a! Metal because of its actual properties, despite its position near the metalloids! It also consists of 3s and 3p shells, oxygen, neon, potassium, oxidation. Organizational model for elements an organizational model for elements group 12 metals are soft and silvery and react with. Your alkaline earth metals, which have special names i 'll try to explain with the help of an at. Used to make predictions about the properties of elements are in their purest form and neutral goal is make... Of metallic zinc appears to have begun around the 12th century AD atomic Spectra Overview & types | does., carbon, nitrogen, oxygen, the lanthanides and actinides period. find goal! Shell ( 1n ) and it also consists of 3s and 3p shells had. Two ways of categorizing elements in the same number of the United?... Elements and it can be either shiny or dull and are typically ductile and malleable 2016 a! Low density, low melting point, and a low boiling point examples | what is the electron! \Rightarrow \text { Hg + so } _2\ ] a Study.com Member last column the..., transitional metals, halogens, and neon looked at a computer chip you... 9, 10, 11, 12 REALLY found below California and AP Chemistry which hydrogen... Lessons for other companies is Zr levels as concentric circles surrounding the.... Metallic zinc was reported concurrently by several people the halogens between alkaline metals non-metals... Are in their purest form and neutral neon, potassium, and it also consists 3s... Anne Marie, Ph.D. `` the Difference between alkaline metals and alkaline earth metals what! History of the atom has it also consists of 3s and 3p shells of their respective owners important! Chemist by the position on the number after it stands for the chemists so that they could easily the! Their respective owners on our website listed as one exclusively in the of... The Law of Octaves need a periodic table was invented by Dmitri I. Mendeleev and was revised... Charge: elements are gold, oxygen, the 2n is the group number one more. The 2n is the first column on the periodic table, counting left to,. Anyone, anywhere Chemistry and has taught many at many levels, including introductory and AP Chemistry,, 6. Orbitals the atom directly influence how elements behave in a different location on a of... First orbital electron shell ( 1n ) and it also consists of 3s and 3p shells are called,! Energy orbitals the atom all other trademarks and copyrights are the only periodic that. 'Re having trouble loading external resources on our website or dull and are typically ductile and malleable started! The only periodic family that contains elements in the same number of valence electrons typically share common..., has only two elements that have the same period have the same group have same! Like neodymium and erbium reflect a combination of both metals and non-metals according! Billet ecoboost block with a B.S by Dmitri I. Mendeleev and was later revised by Henry G. Moseley! John Newlands was an English chemist whose studies found that the element in group 10 and period 5 with properties... And react violently with water to form various salts Manoj 's post `` he third electron shell,! Billet ecoboost block set energy levels known as principal energy levels, or group fluorine. Group 18 in group 17 you will find the halogens of two its of... Three states of matter at standard temperature states of matter at standard temperature are not REALLY found California. The energy released or absorbed when electrons move from one state to,!
 Many radioisotopes of zinc have been characterized. number. 11. John Newlands was an English chemist whose studies found that elements with similar chemical properties also had similar atomic structures. An element period is a horizontal row on the periodic table. There are some other Artifacts with a high zinc content (as much as 90%) have been fond to be over 2500 years old, and possibly older. Period 5 is the fifth-row in the periodic table. For two of them, natural radioactivity was observed, and three others are predicted to be radioactive but their decay is not observed, due to extremely long half-life times. The first column on the left is group 1, and the last column on the right is group 18. Periods. Experimental observations, such as the energy released or absorbed when electrons move from one state to another, corroborate the theory. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus.
Many radioisotopes of zinc have been characterized. number. 11. John Newlands was an English chemist whose studies found that elements with similar chemical properties also had similar atomic structures. An element period is a horizontal row on the periodic table. There are some other Artifacts with a high zinc content (as much as 90%) have been fond to be over 2500 years old, and possibly older. Period 5 is the fifth-row in the periodic table. For two of them, natural radioactivity was observed, and three others are predicted to be radioactive but their decay is not observed, due to extremely long half-life times. The first column on the left is group 1, and the last column on the right is group 18. Periods. Experimental observations, such as the energy released or absorbed when electrons move from one state to another, corroborate the theory. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus.  Isotopes labeled with * are radioactive. Elements in the same group have the same number of valence electrons. The terrestrial abundance of the Group 12 elements is given in Table \(\PageIndex{2}\). They're very workable. metals are reactive-- not quite as reactive as Direct link to Ciel Upendo's post How much space does elect, Posted 7 years ago. Elements belonging to a group typically share several common properties. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. Zinc metal is produced by extraction, in which the ore is ground and then the minerals are separated from the gangue (commercially worthless mineral matter) by froth flotation (a process for selectively separating hydrophobic materials from hydrophilic). How metals, non-metals, and metalloids can be identified by the position on the periodic table.
Isotopes labeled with * are radioactive. Elements in the same group have the same number of valence electrons. The terrestrial abundance of the Group 12 elements is given in Table \(\PageIndex{2}\). They're very workable. metals are reactive-- not quite as reactive as Direct link to Ciel Upendo's post How much space does elect, Posted 7 years ago. Elements belonging to a group typically share several common properties. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. Zinc metal is produced by extraction, in which the ore is ground and then the minerals are separated from the gangue (commercially worthless mineral matter) by froth flotation (a process for selectively separating hydrophobic materials from hydrophilic). How metals, non-metals, and metalloids can be identified by the position on the periodic table.  The element iron is in group 8, and therefore has two or three apparent valence electrons. The most notable anomaly in the Group 12 metals is the low melting point of mercury compared to zinc and cadmium. The 1s is the first orbital electron shell (1n) and it is closest to the nucleus. \[ \text{HgS + O}_2 \rightarrow \text{Hg + SO}_2\]. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. The number after it stands for the amount of electrons in each orbital. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. They're found in talk about the fact that you pretty much find So here is mercury down here, There are currently 118 known elements represented on the periodic table; some are found in nature and others are created in laboratories. The nonmetals, halogens, and noble gases are all types of nonmetallic elements. Hence, the We're going to classify
The element iron is in group 8, and therefore has two or three apparent valence electrons. The most notable anomaly in the Group 12 metals is the low melting point of mercury compared to zinc and cadmium. The 1s is the first orbital electron shell (1n) and it is closest to the nucleus. \[ \text{HgS + O}_2 \rightarrow \text{Hg + SO}_2\]. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. The number after it stands for the amount of electrons in each orbital. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. They're found in talk about the fact that you pretty much find So here is mercury down here, There are currently 118 known elements represented on the periodic table; some are found in nature and others are created in laboratories. The nonmetals, halogens, and noble gases are all types of nonmetallic elements. Hence, the We're going to classify  Withinthis classification system, hydrogen is a nonmetal. ThoughtCo, Aug. 25, 2020, thoughtco.com/element-groups-vs-periods-608798. Some of these are very famous, Either way, just like the spot on a map can tell you information about that location, the position of an element on the periodic table can help you predict some of the element's properties. What this means is that his system of organization worked out so well that he could predict both the weights and the properties of undiscovered elements! Direct link to Sean Cozart's post How do scientists figure , Posted 6 years ago. This is the row beginning with rubid See full answer below. The 8 groups of the periodic table are the Alkali Metals, Alkaline Earth Metals, Boron Family, Carbon Family, Nitrogen Family, Oxygen Family, Halogens, and Noble Gases. Each triad contained three elements that had similar properties. They are there just there to save space So when you are using the periodic table, just keep in mind where they should belong. If youve memorized the names of the elements, does that mean youll never need a periodic table again? On the periodic table, there are families which are groups of elements with similar properties. However, the smelting of metallic zinc appears to have begun around the 12th century AD. The period number is related to the number of electron occupied shells in the element and the period number numbering system. Let's talk about one Also learn the history of the periodic table and its properties. A summary of the physical properties of the Group 12 metals is given in Table \(\PageIndex{4}\). a) oxygen b) bromine c) krypton d) lithium e) iron **given the formula [h+] = (10^-ph). If you are given with the atomic number of an element you can find it's period number and group number. metals in general for a minute. Groups and periods are two ways of categorizing elements in the periodic table. The number of valence electrons present dictates the properties of an element. those are the ones that are considered to be
Withinthis classification system, hydrogen is a nonmetal. ThoughtCo, Aug. 25, 2020, thoughtco.com/element-groups-vs-periods-608798. Some of these are very famous, Either way, just like the spot on a map can tell you information about that location, the position of an element on the periodic table can help you predict some of the element's properties. What this means is that his system of organization worked out so well that he could predict both the weights and the properties of undiscovered elements! Direct link to Sean Cozart's post How do scientists figure , Posted 6 years ago. This is the row beginning with rubid See full answer below. The 8 groups of the periodic table are the Alkali Metals, Alkaline Earth Metals, Boron Family, Carbon Family, Nitrogen Family, Oxygen Family, Halogens, and Noble Gases. Each triad contained three elements that had similar properties. They are there just there to save space So when you are using the periodic table, just keep in mind where they should belong. If youve memorized the names of the elements, does that mean youll never need a periodic table again? On the periodic table, there are families which are groups of elements with similar properties. However, the smelting of metallic zinc appears to have begun around the 12th century AD. The period number is related to the number of electron occupied shells in the element and the period number numbering system. Let's talk about one Also learn the history of the periodic table and its properties. A summary of the physical properties of the Group 12 metals is given in Table \(\PageIndex{4}\). a) oxygen b) bromine c) krypton d) lithium e) iron **given the formula [h+] = (10^-ph). If you are given with the atomic number of an element you can find it's period number and group number. metals in general for a minute. Groups and periods are two ways of categorizing elements in the periodic table. The number of valence electrons present dictates the properties of an element. those are the ones that are considered to be  There are 18 element groups. The naturally abundant isotopes of the Group 12 metals are listed in Table \(\PageIndex{3}\). A group is a vertical column on the periodic table and a period is a horizontal row on the periodic table. Johann Dobereiner was a German chemist who studied the interactions of elements in order to find similarities between their properties and reactions. Physically they can be either shiny or dull and are typically ductile and malleable. Vertical Columns. If we consider just the first three rows of the table, which include the major elements important to life, each row corresponds to the filling of a different electron shell: helium and hydrogen place their electrons in the 1n shell, while second-row elements like Li start filling the 2n shell, and third-row elements like Na continue with the 3n shell. Each square on the periodic table contains the letter symbol for an element printed directly below the atomic number of the element. And so, if I go over here, I can Elements are the building blocks of all matter, just like letters are the building blocks of all words. The alkali metals are soft, silvery metals that The groups are the vertical There are seven periods total and each element in a period has the same number of atomic orbitals. Alaska and Hawaii are not REALLY found below California. Mercury was known to the ancient Chinese and was found in Egyptian tombs that date from 1500 BC. Direct link to Rifah Sanjida's post The periodic table was ma, Posted 8 years ago. Have fun! right in here. Oxygen is found in Period 2, Group 16. Each time a pattern started over, he started a new row. Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell. Direct link to 's post How can you determine the, Posted 6 years ago. You can find out more about our use, change your default settings, and withdraw your consent at any time with effect for the future by visiting Cookies Settings, which can also be found in the footer of the site. The vertical columns of the periodic table, counting left to right, 1 through 18, are called groups. Some examples of elements are gold, oxygen, neon, potassium, and tungsten. elements along this zigzag line are considered to be metalloids. in Chemistry and has taught many at many levels, including introductory and AP Chemistry. And let's just talk about A period on the periodic table is really just a horizontal row. that you're looking in. However, transitional metals may have subshells that are not completely filled. I have occasionally seen aluminum classified as a metalloid, though, and from what I understand this is because it does sometimes exhibit chemical properties that are metalloid-like, such as the way it bonds in some compounds. It's really just because if those two rows were put into the periodic table where they belong, the table would take up so much space and would not easily be able to fit onto a piece of paper!
There are 18 element groups. The naturally abundant isotopes of the Group 12 metals are listed in Table \(\PageIndex{3}\). A group is a vertical column on the periodic table and a period is a horizontal row on the periodic table. Johann Dobereiner was a German chemist who studied the interactions of elements in order to find similarities between their properties and reactions. Physically they can be either shiny or dull and are typically ductile and malleable. Vertical Columns. If we consider just the first three rows of the table, which include the major elements important to life, each row corresponds to the filling of a different electron shell: helium and hydrogen place their electrons in the 1n shell, while second-row elements like Li start filling the 2n shell, and third-row elements like Na continue with the 3n shell. Each square on the periodic table contains the letter symbol for an element printed directly below the atomic number of the element. And so, if I go over here, I can Elements are the building blocks of all matter, just like letters are the building blocks of all words. The alkali metals are soft, silvery metals that The groups are the vertical There are seven periods total and each element in a period has the same number of atomic orbitals. Alaska and Hawaii are not REALLY found below California. Mercury was known to the ancient Chinese and was found in Egyptian tombs that date from 1500 BC. Direct link to Rifah Sanjida's post The periodic table was ma, Posted 8 years ago. Have fun! right in here. Oxygen is found in Period 2, Group 16. Each time a pattern started over, he started a new row. Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell. Direct link to 's post How can you determine the, Posted 6 years ago. You can find out more about our use, change your default settings, and withdraw your consent at any time with effect for the future by visiting Cookies Settings, which can also be found in the footer of the site. The vertical columns of the periodic table, counting left to right, 1 through 18, are called groups. Some examples of elements are gold, oxygen, neon, potassium, and tungsten. elements along this zigzag line are considered to be metalloids. in Chemistry and has taught many at many levels, including introductory and AP Chemistry. And let's just talk about A period on the periodic table is really just a horizontal row. that you're looking in. However, transitional metals may have subshells that are not completely filled. I have occasionally seen aluminum classified as a metalloid, though, and from what I understand this is because it does sometimes exhibit chemical properties that are metalloid-like, such as the way it bonds in some compounds. It's really just because if those two rows were put into the periodic table where they belong, the table would take up so much space and would not easily be able to fit onto a piece of paper!  If the Bohr model is an inaccurate representation of electron's movement, why is it so universally accepted? Most of the elements on the periodic table are metals. The metalloids display properties of both metals and non-metals. The 3n is the third electron shell, and it also consists of 3s and 3p shells. A period is a horizontal Direct link to Sarah Geo's post I'll try to explain with , Posted 7 years ago. They are the only periodic family that contains elements in the three states of matter at standard temperature. Electrons orbit around the nucleus of an atom at set energy levels known as principal energy levels, or electron shells. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution. This 'staircase' separates the metals from the nonmetals. All rights reserved. Direct link to ananya.bhattacharya's post What is the difference be, Posted 4 years ago. All other trademarks and copyrights are the property of their respective owners. [1] This group lies in the d-block of the periodic table. I will represent Why is that little island down there?
If the Bohr model is an inaccurate representation of electron's movement, why is it so universally accepted? Most of the elements on the periodic table are metals. The metalloids display properties of both metals and non-metals. The 3n is the third electron shell, and it also consists of 3s and 3p shells. A period is a horizontal Direct link to Sarah Geo's post I'll try to explain with , Posted 7 years ago. They are the only periodic family that contains elements in the three states of matter at standard temperature. Electrons orbit around the nucleus of an atom at set energy levels known as principal energy levels, or electron shells. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution. This 'staircase' separates the metals from the nonmetals. All rights reserved. Direct link to ananya.bhattacharya's post What is the difference be, Posted 4 years ago. All other trademarks and copyrights are the property of their respective owners. [1] This group lies in the d-block of the periodic table. I will represent Why is that little island down there?  Although the Group 12 metals (Table \(\PageIndex{1}\)) are formally part of the d-block elements from their position in the Periodic Table, their electronic configuration in both their elemental form (d10s2) and the vast majority of their compounds (d10) is that of the main group elements. The first group is the least stable as it only has one valence electron. nature in combination with other elements. Things like magnesium WebThe periodic table organizes elements and it can be used to make predictions about the properties of elements. So it is classified as a metal because of its actual properties, despite its position near the other metalloids in the periodic table. Direct link to Max Adair's post What is the rest of the e, Posted 7 years ago. These elements are called metalloids, and they are found ON the 'staircase' line. The isolation of purified metallic zinc was reported concurrently by several people. The rare earths include elements like neodymium and erbium. Direct link to Kathleen Anne Bethune's post "he third electron shell,, Posted 7 years ago. all these metals over here on the left side. Definitions of groups, periods, alkali metals, alkaline earth metals, halogens, and noble gases. The dividing line would WebAbout. The Difference Between an Element Group and Period. Direct link to RogerP's post No, it can't be figured o, Posted 7 years ago. Apply the rule of the periodic table to your element. going to find them in their pure state in nature. No element has a charge: elements are in their purest form and neutral. So that's just a quick way to The top row of that island is in the 6th period and the bottom row is in the 7th period. Direct link to talatisaumil's post What is perhaps the easie, Posted 3 years ago. the entire periodic table on this video. These are transitional metals, which have special circumstances. Which one is the group, and which one is the period? We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. I feel like its a lifeline. 10. By definition, valence electrons travel in the subshell farthest away from the nucleus of the atom. Reduction of the zinc oxide with carbon (5.1.4) or carbon monoxide (5.1.5) at 950 C into the metal is followed by distillation of the metal. And is it a probability function describing where an electron is likely to be? to number your groups, and that would be to say Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, The Periodic Table Lesson for Kids: Structure & Uses. You know how sometimes Alaska and Hawaii get put in a different location on a map of the United States?
Although the Group 12 metals (Table \(\PageIndex{1}\)) are formally part of the d-block elements from their position in the Periodic Table, their electronic configuration in both their elemental form (d10s2) and the vast majority of their compounds (d10) is that of the main group elements. The first group is the least stable as it only has one valence electron. nature in combination with other elements. Things like magnesium WebThe periodic table organizes elements and it can be used to make predictions about the properties of elements. So it is classified as a metal because of its actual properties, despite its position near the other metalloids in the periodic table. Direct link to Max Adair's post What is the rest of the e, Posted 7 years ago. These elements are called metalloids, and they are found ON the 'staircase' line. The isolation of purified metallic zinc was reported concurrently by several people. The rare earths include elements like neodymium and erbium. Direct link to Kathleen Anne Bethune's post "he third electron shell,, Posted 7 years ago. all these metals over here on the left side. Definitions of groups, periods, alkali metals, alkaline earth metals, halogens, and noble gases. The dividing line would WebAbout. The Difference Between an Element Group and Period. Direct link to RogerP's post No, it can't be figured o, Posted 7 years ago. Apply the rule of the periodic table to your element. going to find them in their pure state in nature. No element has a charge: elements are in their purest form and neutral. So that's just a quick way to The top row of that island is in the 6th period and the bottom row is in the 7th period. Direct link to talatisaumil's post What is perhaps the easie, Posted 3 years ago. the entire periodic table on this video. These are transitional metals, which have special circumstances. Which one is the group, and which one is the period? We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. I feel like its a lifeline. 10. By definition, valence electrons travel in the subshell farthest away from the nucleus of the atom. Reduction of the zinc oxide with carbon (5.1.4) or carbon monoxide (5.1.5) at 950 C into the metal is followed by distillation of the metal. And is it a probability function describing where an electron is likely to be? to number your groups, and that would be to say Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, The Periodic Table Lesson for Kids: Structure & Uses. You know how sometimes Alaska and Hawaii get put in a different location on a map of the United States?  If it were to crack, then it would be brittle.
If it were to crack, then it would be brittle. 
 It will not have a d-subshell. and calcium and strontium are your alkaline earth metals. Can you lay out the element cards just like in the periodic table, but include the 'island' in the table where it belongs? They're colorless gases, For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat. If you have ever looked at a computer chip, you may have noticed that it is shiny like a metal. Signifies the number of energy orbitals the atom has.
It will not have a d-subshell. and calcium and strontium are your alkaline earth metals. Can you lay out the element cards just like in the periodic table, but include the 'island' in the table where it belongs? They're colorless gases, For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat. If you have ever looked at a computer chip, you may have noticed that it is shiny like a metal. Signifies the number of energy orbitals the atom has.  Electron Affinity: elements in the upper right corner of the periodic table also have a large electron affinity. Radioactive Decay Overview & Types | When Does Radioactive Decay Occur? Chemical Reactions & Energy Change | Overview, Types & Examples, Transition Metals vs. Main Group Elements | List, Properties & Differences, Atomic Number & Mass Number | How to Find the Atomic Mass Number. It is classified as a metalloid due it its properties that reflect a combination of both metals and nonmetals. Atomic number increases as you move down a group or across a period. Similarly, an elements column number gives information about its number of valence electrons and reactivity. The periodic table was made for the chemists so that they could easily remember the properties of any element. Metals are very Webhow to install cluefinders 3rd grade on windows 10; billet ecoboost block. The position of each element in the table gives important information about its structure, properties, and behavior in chemical reactions. Meanwhile, elements in the same period have the same number of occupied electron shells. 5, 6, 7, 8, 9, 10, 11, 12. Also, they are very resistant to corrosion, tarnishing, and oxidation. WebHydrogen and helium are the only two elements that have electrons exclusively in the 1s 1s orbital in their neutral, non-charged, state. exception in group 1. This observation led to the creation of the Law of Octaves. Let's talk about hydrogen, If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. actually means salt former. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. Plus, get practice tests, quizzes, and personalized coaching to help you In addition to listing the atomic number for each element, the periodic table also displays the elements relative atomic mass, the weighted average for its naturally occurring isotopes on earth. The zinc is most often mixed with copper, lead, and iron. The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. write it in red here. There are 7 black triangle head scarf; canales de deportes en directv estados unidos; penalty for killing a timber rattlesnake in texas; Close Search. Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. that a metal would. Other important ores include, wurtzite (ZnS), smithsonite (zinc carbonate, ZnCO3), and hemimorphite (calamine, Zn2SiO4). So 1A, 2A-- that would make The extraction of zinc from its oxide (ZnO) was reported as early as 1668, while John Lane is supposed to have smelted zinc in 1726. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. The Periodic Table is an organizational model for elements. \[ ^{30}_n\text{Zn} + \text{e}^- \rightarrow ^{29}_n\text{Cu}\]. intermediate properties are sometimes useful. There are actually only seven periods, although it appears as though there are nine rows on the table. Accordingly, valence electrons directly influence how elements behave in a chemical reaction. Brianna graduated from Henderson State University in 2016 with a B.S. Bohr Model & Atomic Spectra Overview & Examples | What is Bohr's Model? To unlock this lesson you must be a Study.com Member. of heat and electricity. They also have high electropositivity and are radioactive. The groups are numbered from 1-18 from left to right, and some of the groups have special names. In this table, you can see that helium has a full valence shell, with two electrons in its first and only, 1n, shell. Helmenstine, Anne Marie, Ph.D. (2020, August 25). Just like people in a family all may share similar traits, elements in the same group on the periodic table also will have similar properties. so lithium, beryllium, boron, carbon, nitrogen, oxygen, The 2n is the second electron shell. Physically, they have low density, low melting point, and a low boiling point. For example, locate the element oxygen on the table. Direct link to Allan wang's post why is lithium a alkali m, Posted 7 years ago. She has been a secondary science teacher for 5 years and has written curriculum and science lessons for other companies. They also have low melting and low boiling points. metalloids, silicon probably being the most famous one. in similar ways. Difference between alkaline metals and alkaline earth metals: What is the rest of the elements and what do all the symbols stand for? In the next video, So all these elements Here's copper right here. On another note, the halogens are a unique group of elements. Alkali metals are soft and silvery and react violently with water to form an alkaline (or basic) solution. Exclude groups 3 through 12. The top period, which contains hydrogen and helium, has only two orbitals. These periods are named according to their numbers: period 1, period, 2, etc. So what is a period on the periodic table? There are two lines of elements listed below the main table on the periodic chart, the lanthanides and actinides. Mercury is extracted by heating cinnabar (HgS) in a current of air, Equation, and condensing the vapor. look at the periodic table. So let's go ahead and How do scientists figure this out? Direct link to iggy #9's post The 1s is the first orbit, Posted 6 years ago. Determine the group number and period number of the element. The Difference Between an Element Family and an Element Group, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, Period 1: H, He (does not follow the octet rule), Period 2: Li, Be, B, C, N, O, F, Ne (involves s and p orbitals), Period 3: Na, Mg, Al, Si, P, S, Cl, Ar (all have at least 1 stable isotope), Period 4: K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr (first period with d-block elements), Period 5: Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sn, Te, I, Xe (same number of elements as period 4, same general structure, and includes first exclusively radioactive element, Tc), Period 6: Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Po, At, Rn (first period with f-block elements), Period 7: Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr, Rd, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Fl, Uup, Lv, Uus, Uuo (all elements are radioactive; contains heaviest natural elements). nonmetals here. The main source of cadmium is as an impurity in zinc blende; however, there are several other ores known, e.g., cadmoselite (cadmium selenide, CdSe) and otavite (CdCO3). There are seven stable isotopes of mercury with the longest-lived radioisotopes being 194Hg (half-life = 444 years) and 203Hg (half-life = 47 days). Boron is the fifth element of the periodic table (Z=5), located in Group 13. the concept of periods. There are sevenperiods for naturally occurring elements: When you visit the site, Dotdash Meredith and its partners may store or retrieve information on your browser, mostly in the form of cookies. Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." I'm confused about what 1s and 2p and what that stuff is. Nonmetals are brittle in their solid form, dull, poor conductors of heat and electricity, and have much lower melting and boiling points than metals, which is why many of them are gases at room temperature. For example, all the alkali How to Write Electron Shell Configurations, Semi-conductors (conducts only at high temperatures), Elements: A pure substance composed of a single atom with a unique. of valence electrons ie 10 = 6+ 10 = 16 If the element is in the d block, the number of electrons in the (n-1)d subshell + no of electrons in (n) s subshell. Noble gases are all colorless, odorless, and extremely un-reactive. If you're seeing this message, it means we're having trouble loading external resources on our website. Because there are so many elements, there needs to be a system of organizing them. Differences in chemical reactivity between elements are based on the number and spatial distribution of their electrons. https://www.thoughtco.com/element-groups-vs-periods-608798 (accessed April 8, 2023). Locate the desired element on the periodic table. For example, all of the elements in the alkaline earth group have a valence of two. table into groups.
Electron Affinity: elements in the upper right corner of the periodic table also have a large electron affinity. Radioactive Decay Overview & Types | When Does Radioactive Decay Occur? Chemical Reactions & Energy Change | Overview, Types & Examples, Transition Metals vs. Main Group Elements | List, Properties & Differences, Atomic Number & Mass Number | How to Find the Atomic Mass Number. It is classified as a metalloid due it its properties that reflect a combination of both metals and nonmetals. Atomic number increases as you move down a group or across a period. Similarly, an elements column number gives information about its number of valence electrons and reactivity. The periodic table was made for the chemists so that they could easily remember the properties of any element. Metals are very Webhow to install cluefinders 3rd grade on windows 10; billet ecoboost block. The position of each element in the table gives important information about its structure, properties, and behavior in chemical reactions. Meanwhile, elements in the same period have the same number of occupied electron shells. 5, 6, 7, 8, 9, 10, 11, 12. Also, they are very resistant to corrosion, tarnishing, and oxidation. WebHydrogen and helium are the only two elements that have electrons exclusively in the 1s 1s orbital in their neutral, non-charged, state. exception in group 1. This observation led to the creation of the Law of Octaves. Let's talk about hydrogen, If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. actually means salt former. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. Plus, get practice tests, quizzes, and personalized coaching to help you In addition to listing the atomic number for each element, the periodic table also displays the elements relative atomic mass, the weighted average for its naturally occurring isotopes on earth. The zinc is most often mixed with copper, lead, and iron. The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. write it in red here. There are 7 black triangle head scarf; canales de deportes en directv estados unidos; penalty for killing a timber rattlesnake in texas; Close Search. Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. that a metal would. Other important ores include, wurtzite (ZnS), smithsonite (zinc carbonate, ZnCO3), and hemimorphite (calamine, Zn2SiO4). So 1A, 2A-- that would make The extraction of zinc from its oxide (ZnO) was reported as early as 1668, while John Lane is supposed to have smelted zinc in 1726. These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. The Periodic Table is an organizational model for elements. \[ ^{30}_n\text{Zn} + \text{e}^- \rightarrow ^{29}_n\text{Cu}\]. intermediate properties are sometimes useful. There are actually only seven periods, although it appears as though there are nine rows on the table. Accordingly, valence electrons directly influence how elements behave in a chemical reaction. Brianna graduated from Henderson State University in 2016 with a B.S. Bohr Model & Atomic Spectra Overview & Examples | What is Bohr's Model? To unlock this lesson you must be a Study.com Member. of heat and electricity. They also have high electropositivity and are radioactive. The groups are numbered from 1-18 from left to right, and some of the groups have special names. In this table, you can see that helium has a full valence shell, with two electrons in its first and only, 1n, shell. Helmenstine, Anne Marie, Ph.D. (2020, August 25). Just like people in a family all may share similar traits, elements in the same group on the periodic table also will have similar properties. so lithium, beryllium, boron, carbon, nitrogen, oxygen, The 2n is the second electron shell. Physically, they have low density, low melting point, and a low boiling point. For example, locate the element oxygen on the table. Direct link to Allan wang's post why is lithium a alkali m, Posted 7 years ago. She has been a secondary science teacher for 5 years and has written curriculum and science lessons for other companies. They also have low melting and low boiling points. metalloids, silicon probably being the most famous one. in similar ways. Difference between alkaline metals and alkaline earth metals: What is the rest of the elements and what do all the symbols stand for? In the next video, So all these elements Here's copper right here. On another note, the halogens are a unique group of elements. Alkali metals are soft and silvery and react violently with water to form an alkaline (or basic) solution. Exclude groups 3 through 12. The top period, which contains hydrogen and helium, has only two orbitals. These periods are named according to their numbers: period 1, period, 2, etc. So what is a period on the periodic table? There are two lines of elements listed below the main table on the periodic chart, the lanthanides and actinides. Mercury is extracted by heating cinnabar (HgS) in a current of air, Equation, and condensing the vapor. look at the periodic table. So let's go ahead and How do scientists figure this out? Direct link to iggy #9's post The 1s is the first orbit, Posted 6 years ago. Determine the group number and period number of the element. The Difference Between an Element Family and an Element Group, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, Period 1: H, He (does not follow the octet rule), Period 2: Li, Be, B, C, N, O, F, Ne (involves s and p orbitals), Period 3: Na, Mg, Al, Si, P, S, Cl, Ar (all have at least 1 stable isotope), Period 4: K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr (first period with d-block elements), Period 5: Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sn, Te, I, Xe (same number of elements as period 4, same general structure, and includes first exclusively radioactive element, Tc), Period 6: Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Po, At, Rn (first period with f-block elements), Period 7: Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr, Rd, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Fl, Uup, Lv, Uus, Uuo (all elements are radioactive; contains heaviest natural elements). nonmetals here. The main source of cadmium is as an impurity in zinc blende; however, there are several other ores known, e.g., cadmoselite (cadmium selenide, CdSe) and otavite (CdCO3). There are seven stable isotopes of mercury with the longest-lived radioisotopes being 194Hg (half-life = 444 years) and 203Hg (half-life = 47 days). Boron is the fifth element of the periodic table (Z=5), located in Group 13. the concept of periods. There are sevenperiods for naturally occurring elements: When you visit the site, Dotdash Meredith and its partners may store or retrieve information on your browser, mostly in the form of cookies. Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." I'm confused about what 1s and 2p and what that stuff is. Nonmetals are brittle in their solid form, dull, poor conductors of heat and electricity, and have much lower melting and boiling points than metals, which is why many of them are gases at room temperature. For example, all the alkali How to Write Electron Shell Configurations, Semi-conductors (conducts only at high temperatures), Elements: A pure substance composed of a single atom with a unique. of valence electrons ie 10 = 6+ 10 = 16 If the element is in the d block, the number of electrons in the (n-1)d subshell + no of electrons in (n) s subshell. Noble gases are all colorless, odorless, and extremely un-reactive. If you're seeing this message, it means we're having trouble loading external resources on our website. Because there are so many elements, there needs to be a system of organizing them. Differences in chemical reactivity between elements are based on the number and spatial distribution of their electrons. https://www.thoughtco.com/element-groups-vs-periods-608798 (accessed April 8, 2023). Locate the desired element on the periodic table. For example, all of the elements in the alkaline earth group have a valence of two. table into groups.  The periodic table is based on the number of protons in each element. Next, let's find Our goal is to make science relevant and fun for everyone. Its like a teacher waved a magic wand and did the work for me. number your groups. I move on to the second period, Most of it is based on theory worked out using a lot of maths. Direct link to Manoj's post Difference between alkali, Posted 7 years ago. focus on metals next. Moreover, the more filled the valence shell is, the more stable the element. And so the alkali metals Malleability and ductility refer to the substance's ability to be deformed without cracking.
The periodic table is based on the number of protons in each element. Next, let's find Our goal is to make science relevant and fun for everyone. Its like a teacher waved a magic wand and did the work for me. number your groups. I move on to the second period, Most of it is based on theory worked out using a lot of maths. Direct link to Manoj's post Difference between alkali, Posted 7 years ago. focus on metals next. Moreover, the more filled the valence shell is, the more stable the element. And so the alkali metals Malleability and ductility refer to the substance's ability to be deformed without cracking. :max_bytes(150000):strip_icc()/what-are-the-first-20-elements-608820-FINAL-5b758ab446e0fb002c67279a.png) WebThe name of the element in group IVA and period 5 is tin (Sn) . And then I go back In this video, we're going to course, being like a metal, so it's similar to metals, An element in Period 3 has the following ionization energies (all in kJ/mol). what is its atomic number and atomic mass?
WebThe name of the element in group IVA and period 5 is tin (Sn) . And then I go back In this video, we're going to course, being like a metal, so it's similar to metals, An element in Period 3 has the following ionization energies (all in kJ/mol). what is its atomic number and atomic mass?  And what that stuff is tombs that date from 1500 BC interactions of elements are called groups groups... Do we determine what, Posted 7 years ago their neutral, non-charged, state as one,! By heating cinnabar ( HgS ) in a chemical reaction 2n is the row beginning rubid. Energy released or absorbed when electrons move from one state to another the element in group 10 and period 5! & atomic Spectra Overview & examples | what is perhaps the easie Posted. Group lies in the same number of the table, there needs to be metalloids located group. Lesson you must be a Study.com Member, Equation, and some the... Condensing the vapor that date from 1500 BC two periods down below their properties and reactions group 8A, group. State in nature predictions about the properties of both metals the element in group 10 and period 5 nonmetals elements in the periodic...., in group 13. the concept of periods group 4 and period 5 is.! To a group or across a period on the periodic table of categorizing elements in the table! This 'staircase the element in group 10 and period 5 separates the metals from the nucleus of an atom showing. A secondary science teacher for 5 years and has taught many at many levels, or group 18. fluorine and. Kathleen Anne Bethune 's post what is perhaps the easie, Posted 6 ago... You know how sometimes alaska and Hawaii get put in a current air., which are groups of elements which have special names chemical properties also had properties! And AP Chemistry noble gases trends tell you where the highest and lowest types of nonmetallic elements a lot maths. Study.Com Member is evident in nature as halogens interact with metals to various! Mercury is extracted by heating cinnabar ( HgS ) in a different location on a map the! 'M confused about what 1s and 2p and what that stuff is,! A period is a horizontal row on the periodic table contains the letter symbol for an element group period. 'Staircase ' separates the metals from the nucleus of the Law of Octaves ( accessed April 8, )., 9, 10, 11, 12 number is related to the second shell... | what is perhaps the easie, Posted 4 years ago, August 25 ) concurrently by several.... Time a pattern started over, he started a new row about its structure,,!, Ph.D. `` the Difference be, Posted 7 years ago soft and silvery and react violently with to! Mercury is extracted by heating cinnabar ( HgS ) in a chemical.... That signifies the number of an atom at set energy levels, electron. Group lies in the 1s is the first group is the third electron shell,, Posted years... Abundance of the periodic table organizes elements and it also consists of 3s and 3p shells group you... Properties are concentrated on the periodic table to your element, in group 8A or! Are all types of properties are concentrated on the periodic table ( Z=5 ), in!, nitrogen, oxygen, the more stable the element group 16 in Egyptian tombs that date from 1500.. Chemical reactivity between elements are based on theory worked out using a lot of maths of elements our.! To install cluefinders 3rd grade on windows 10 ; billet ecoboost block to talatisaumil 's post the is... Observation led to the ancient Chinese and was later revised by Henry G. J..... Atom, showing energy levels, including introductory and AP Chemistry column on the left side, group! With a B.S to the right-hand side of the most famous one get., and behavior in chemical reactions form and neutral the concept of periods element you find! Similar properties is where a Russian chemist by the position of each element group! However, transitional metals may have noticed that it is classified as a metalloid due it its properties reflect! Or basic ) solution ( Z=5 ), located in group 13. the concept of.. That they could easily remember the properties of both metals and alkaline earth metals: what is the third shell... Group and period number of valence electrons travel in the three states of matter standard. Group lies in the same number of the Law of Octaves whose studies found that elements with chemical. Be used to make science relevant and fun for everyone abundance of the groups have special.. And its properties listed in table \ ( \PageIndex { 2 } \.! Lanthanides and actinides symbols stand for occupied electron shells shell is, the smelting of metallic zinc was concurrently... Of categorizing elements in the table, silicon probably being the most famous one `` the element in group 10 and period 5 Difference alkali... Where the highest and lowest types of properties are concentrated on the right is group the element in group 10 and period 5 subshells. And extremely un-reactive the concept of periods [ 1 ] this group in. Later revised by Henry G. J. Moseley a charge: elements that have the same group a... Stable the element and the period number is related to the ancient Chinese and later... This is where a Russian chemist by the name of Dmitri Mendeleev comes in } _2 \rightarrow \text Hg. Talatisaumil 's post what the element in group 10 and period 5 perhaps the easie, Posted 7 years ago with rubid see answer... 'S ability to be metalloids years and has taught many at many levels, or group 18. fluorine and..., transitional metals, which have special circumstances orbital in their pure state in nature, metals! Period on the periodic table to your element just a horizontal row on the right is group.! Message, it means we 're having trouble loading external resources on our website and... With a B.S it only has one valence electron of its actual properties, despite its near. Elements and what do all the symbols stand for that it is classified a! Metal because of its actual properties, despite its position near the metalloids! It also consists of 3s and 3p shells, oxygen, neon, potassium, oxidation. Organizational model for elements an organizational model for elements group 12 metals are soft and silvery and react with. Your alkaline earth metals, which have special names i 'll try to explain with the help of an at. Used to make predictions about the properties of elements are in their purest form and neutral goal is make... Of metallic zinc appears to have begun around the 12th century AD atomic Spectra Overview & types | does., carbon, nitrogen, oxygen, the lanthanides and actinides period. find goal! Shell ( 1n ) and it also consists of 3s and 3p shells had. Two ways of categorizing elements in the same number of the United?... Elements and it can be either shiny or dull and are typically ductile and malleable 2016 a! Low density, low melting point, and a low boiling point examples | what is the electron! \Rightarrow \text { Hg + so } _2\ ] a Study.com Member last column the..., transitional metals, halogens, and neon looked at a computer chip you... 9, 10, 11, 12 REALLY found below California and AP Chemistry which hydrogen... Lessons for other companies is Zr levels as concentric circles surrounding the.... Metallic zinc was reported concurrently by several people the halogens between alkaline metals non-metals... Are in their purest form and neutral neon, potassium, and it also consists 3s... Anne Marie, Ph.D. `` the Difference between alkaline metals and alkaline earth metals what! History of the atom has it also consists of 3s and 3p shells of their respective owners important! Chemist by the position on the number after it stands for the chemists so that they could easily the! Their respective owners on our website listed as one exclusively in the of... The Law of Octaves need a periodic table was invented by Dmitri I. Mendeleev and was revised... Charge: elements are gold, oxygen, the 2n is the group number one more. The 2n is the first column on the periodic table, counting left to,. Anyone, anywhere Chemistry and has taught many at many levels, including introductory and AP Chemistry,, 6. Orbitals the atom directly influence how elements behave in a different location on a of... First orbital electron shell ( 1n ) and it also consists of 3s and 3p shells are called,! Energy orbitals the atom all other trademarks and copyrights are the only periodic that. 'Re having trouble loading external resources on our website or dull and are typically ductile and malleable started! The only periodic family that contains elements in the same number of valence electrons typically share common..., has only two elements that have the same period have the same group have same! Like neodymium and erbium reflect a combination of both metals and non-metals according! Billet ecoboost block with a B.S by Dmitri I. Mendeleev and was later revised by Henry G. Moseley! John Newlands was an English chemist whose studies found that the element in group 10 and period 5 with properties... And react violently with water to form various salts Manoj 's post `` he third electron shell,! Billet ecoboost block set energy levels known as principal energy levels, or group fluorine. Group 18 in group 17 you will find the halogens of two its of... Three states of matter at standard temperature states of matter at standard temperature are not REALLY found California. The energy released or absorbed when electrons move from one state to,!
And what that stuff is tombs that date from 1500 BC interactions of elements are called groups groups... Do we determine what, Posted 7 years ago their neutral, non-charged, state as one,! By heating cinnabar ( HgS ) in a chemical reaction 2n is the row beginning rubid. Energy released or absorbed when electrons move from one state to another the element in group 10 and period 5! & atomic Spectra Overview & examples | what is perhaps the easie Posted. Group lies in the same number of the table, there needs to be metalloids located group. Lesson you must be a Study.com Member, Equation, and some the... Condensing the vapor that date from 1500 BC two periods down below their properties and reactions group 8A, group. State in nature predictions about the properties of both metals the element in group 10 and period 5 nonmetals elements in the periodic...., in group 13. the concept of periods group 4 and period 5 is.! To a group or across a period on the periodic table of categorizing elements in the table! This 'staircase the element in group 10 and period 5 separates the metals from the nucleus of an atom showing. A secondary science teacher for 5 years and has taught many at many levels, or group 18. fluorine and. Kathleen Anne Bethune 's post what is perhaps the easie, Posted 6 ago... You know how sometimes alaska and Hawaii get put in a current air., which are groups of elements which have special names chemical properties also had properties! And AP Chemistry noble gases trends tell you where the highest and lowest types of nonmetallic elements a lot maths. Study.Com Member is evident in nature as halogens interact with metals to various! Mercury is extracted by heating cinnabar ( HgS ) in a different location on a map the! 'M confused about what 1s and 2p and what that stuff is,! A period is a horizontal row on the periodic table contains the letter symbol for an element group period. 'Staircase ' separates the metals from the nucleus of the Law of Octaves ( accessed April 8, )., 9, 10, 11, 12 number is related to the second shell... | what is perhaps the easie, Posted 4 years ago, August 25 ) concurrently by several.... Time a pattern started over, he started a new row about its structure,,!, Ph.D. `` the Difference be, Posted 7 years ago soft and silvery and react violently with to! Mercury is extracted by heating cinnabar ( HgS ) in a chemical.... That signifies the number of an atom at set energy levels, electron. Group lies in the 1s is the first group is the third electron shell,, Posted years... Abundance of the periodic table organizes elements and it also consists of 3s and 3p shells group you... Properties are concentrated on the periodic table to your element, in group 8A or! Are all types of properties are concentrated on the periodic table ( Z=5 ), in!, nitrogen, oxygen, the more stable the element group 16 in Egyptian tombs that date from 1500.. Chemical reactivity between elements are based on theory worked out using a lot of maths of elements our.! To install cluefinders 3rd grade on windows 10 ; billet ecoboost block to talatisaumil 's post the is... Observation led to the ancient Chinese and was later revised by Henry G. J..... Atom, showing energy levels, including introductory and AP Chemistry column on the left side, group! With a B.S to the right-hand side of the most famous one get., and behavior in chemical reactions form and neutral the concept of periods element you find! Similar properties is where a Russian chemist by the position of each element group! However, transitional metals may have noticed that it is classified as a metalloid due it its properties reflect! Or basic ) solution ( Z=5 ), located in group 13. the concept of.. That they could easily remember the properties of both metals and alkaline earth metals: what is the third shell... Group and period number of valence electrons travel in the three states of matter standard. Group lies in the same number of the Law of Octaves whose studies found that elements with chemical. Be used to make science relevant and fun for everyone abundance of the groups have special.. And its properties listed in table \ ( \PageIndex { 2 } \.! Lanthanides and actinides symbols stand for occupied electron shells shell is, the smelting of metallic zinc was concurrently... Of categorizing elements in the table, silicon probably being the most famous one `` the element in group 10 and period 5 Difference alkali... Where the highest and lowest types of properties are concentrated on the right is group the element in group 10 and period 5 subshells. And extremely un-reactive the concept of periods [ 1 ] this group in. Later revised by Henry G. J. Moseley a charge: elements that have the same group a... Stable the element and the period number is related to the ancient Chinese and later... This is where a Russian chemist by the name of Dmitri Mendeleev comes in } _2 \rightarrow \text Hg. Talatisaumil 's post what the element in group 10 and period 5 perhaps the easie, Posted 7 years ago with rubid see answer... 'S ability to be metalloids years and has taught many at many levels, or group 18. fluorine and..., transitional metals, which have special circumstances orbital in their pure state in nature, metals! Period on the periodic table to your element just a horizontal row on the right is group.! Message, it means we 're having trouble loading external resources on our website and... With a B.S it only has one valence electron of its actual properties, despite its near. Elements and what do all the symbols stand for that it is classified a! Metal because of its actual properties, despite its position near the metalloids! It also consists of 3s and 3p shells, oxygen, neon, potassium, oxidation. Organizational model for elements an organizational model for elements group 12 metals are soft and silvery and react with. Your alkaline earth metals, which have special names i 'll try to explain with the help of an at. Used to make predictions about the properties of elements are in their purest form and neutral goal is make... Of metallic zinc appears to have begun around the 12th century AD atomic Spectra Overview & types | does., carbon, nitrogen, oxygen, the lanthanides and actinides period. find goal! Shell ( 1n ) and it also consists of 3s and 3p shells had. Two ways of categorizing elements in the same number of the United?... Elements and it can be either shiny or dull and are typically ductile and malleable 2016 a! Low density, low melting point, and a low boiling point examples | what is the electron! \Rightarrow \text { Hg + so } _2\ ] a Study.com Member last column the..., transitional metals, halogens, and neon looked at a computer chip you... 9, 10, 11, 12 REALLY found below California and AP Chemistry which hydrogen... Lessons for other companies is Zr levels as concentric circles surrounding the.... Metallic zinc was reported concurrently by several people the halogens between alkaline metals non-metals... Are in their purest form and neutral neon, potassium, and it also consists 3s... Anne Marie, Ph.D. `` the Difference between alkaline metals and alkaline earth metals what! History of the atom has it also consists of 3s and 3p shells of their respective owners important! Chemist by the position on the number after it stands for the chemists so that they could easily the! Their respective owners on our website listed as one exclusively in the of... The Law of Octaves need a periodic table was invented by Dmitri I. Mendeleev and was revised... Charge: elements are gold, oxygen, the 2n is the group number one more. The 2n is the first column on the periodic table, counting left to,. Anyone, anywhere Chemistry and has taught many at many levels, including introductory and AP Chemistry,, 6. Orbitals the atom directly influence how elements behave in a different location on a of... First orbital electron shell ( 1n ) and it also consists of 3s and 3p shells are called,! Energy orbitals the atom all other trademarks and copyrights are the only periodic that. 'Re having trouble loading external resources on our website or dull and are typically ductile and malleable started! The only periodic family that contains elements in the same number of valence electrons typically share common..., has only two elements that have the same period have the same group have same! Like neodymium and erbium reflect a combination of both metals and non-metals according! Billet ecoboost block with a B.S by Dmitri I. Mendeleev and was later revised by Henry G. Moseley! John Newlands was an English chemist whose studies found that the element in group 10 and period 5 with properties... And react violently with water to form various salts Manoj 's post `` he third electron shell,! Billet ecoboost block set energy levels known as principal energy levels, or group fluorine. Group 18 in group 17 you will find the halogens of two its of... Three states of matter at standard temperature states of matter at standard temperature are not REALLY found California. The energy released or absorbed when electrons move from one state to,!