For example, a structure for phosphate that has two double bonds is not acceptable. WebAlkenes contain twice as many hydrogen atoms as carbon atoms. For now, however, concentrate on the three main non-radical examples, as these will account for virtually everything we see until chapter 17. Replicating prefixes are ignored when alphabetizing. For example, n-butane has a higher boiling point (0.5 C [31.1 F]) than isobutane (11.7 C [10.9 F]). How does the use of hydrocarbons affect global warming and climate change? Two other p orbitals are available for pi bonding, and a typical compound is the acetylene or ethyne HCCH. Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus. The structure shown below is a line drawing of noncyclic AMP, an important messenger mol- ecule in molecular communication systems. These two perpendicular pairs of p orbitals form two pi bonds between the carbons, resulting in a triple bond overall (one sigma bond plus two pi bonds). Later, we will see how the concept of formal charge can help us to visualize how organic molecules react. Taken from Hybrid Orbitals in Carbon Compounds. a. Step 3) Add electrons to the outer atoms, to complete their octets. Using Lewis structures, we can represent this as follows: Two fluorine atoms can form a molecule of F2 in the same fashion.  Just follow the rules for drawing Lewis structures, and youll get them right! The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). The carbon atoms in an aromatic ring are sp2 hybridized, thus bonding geometry is trigonal planar: in other words, the bonds coming out of the ring are in the same plane as the ring, not pointing above the plane of the ring as the wedges in the incorrect drawing indicate. Write the chemical formula for a molecule of noncyclic AMP. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. James Armstrong, City College of San Francisco, Torrey Glenn, City College of San Francisco. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Nonbonding electrons are always shown using dots. There are often many different possible structures for one molecular formula. The second structure requires more work. Hydrocarbons are the principal constituents of petroleum and natural gas. Corrections? A Lewis structure is a way to show how atoms share electrons when they form a molecule. For each of the hydrogens in methanol, we also get a formal charge of zero: Now, let's look at the cationic form of methanol, CH3OH2+.
Just follow the rules for drawing Lewis structures, and youll get them right! The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). The carbon atoms in an aromatic ring are sp2 hybridized, thus bonding geometry is trigonal planar: in other words, the bonds coming out of the ring are in the same plane as the ring, not pointing above the plane of the ring as the wedges in the incorrect drawing indicate. Write the chemical formula for a molecule of noncyclic AMP. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. James Armstrong, City College of San Francisco, Torrey Glenn, City College of San Francisco. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Nonbonding electrons are always shown using dots. There are often many different possible structures for one molecular formula. The second structure requires more work. Hydrocarbons are the principal constituents of petroleum and natural gas. Corrections? A Lewis structure is a way to show how atoms share electrons when they form a molecule. For each of the hydrogens in methanol, we also get a formal charge of zero: Now, let's look at the cationic form of methanol, CH3OH2+.  They are unambiguous in the sense that, although a single compound may have more than one correct IUPAC name, there is no possibility that two different compounds will have the same name. The C-N sigma bond is an overlap between two sp3 orbitals. Draw the structures of the alkene and the alcohol that react to give the following ether as the major organic product. Of bond e? It MATCHES! Do this for the structures below. are used, along with a separate locant for each substituent. Draw the missing hydrogen atom labels. Youll only see the first four of them in chemical compounds; the last two are extremely radioactive. However, many textbooks (and websites) insist that the structure below is a better one, even though the phosphorus atom has ten electrons around it: The first structure follows the rules for drawing structures. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Clearly, you need to develop the ability to quickly and efficiently draw large structures and determine formal charges. Draw the missing carbon and hydrogen atoms on the molecule. WebDraw the missing carbon and hydrogen atoms on the molecule. For the arrangement HCN,the Lewis structure: HC\(\equiv\)N: For the arrangement HNC,the Lewis structure: HN\(\equiv\)C: Both Lewis structures have a net formal charge of zero, but note thatthe formal charges on the first structure are all zero! Thus the first Lewis structureis predicted to be more stable, and it is, in fact, the structure observed experimentally. COOH HO. NH OH OH b. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. What kind of orbitals overlap to form the C-Cl bonds in chloroform, CHCl3? The bonding has given diamond some very unusual properties. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. The outer atoms are the oxygen atoms here. How does this bonding picture extend to compounds containing carbon-carbon bonds?
They are unambiguous in the sense that, although a single compound may have more than one correct IUPAC name, there is no possibility that two different compounds will have the same name. The C-N sigma bond is an overlap between two sp3 orbitals. Draw the structures of the alkene and the alcohol that react to give the following ether as the major organic product. Of bond e? It MATCHES! Do this for the structures below. are used, along with a separate locant for each substituent. Draw the missing hydrogen atom labels. Youll only see the first four of them in chemical compounds; the last two are extremely radioactive. However, many textbooks (and websites) insist that the structure below is a better one, even though the phosphorus atom has ten electrons around it: The first structure follows the rules for drawing structures. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Clearly, you need to develop the ability to quickly and efficiently draw large structures and determine formal charges. Draw the missing carbon and hydrogen atoms on the molecule. WebDraw the missing carbon and hydrogen atoms on the molecule. For the arrangement HCN,the Lewis structure: HC\(\equiv\)N: For the arrangement HNC,the Lewis structure: HN\(\equiv\)C: Both Lewis structures have a net formal charge of zero, but note thatthe formal charges on the first structure are all zero! Thus the first Lewis structureis predicted to be more stable, and it is, in fact, the structure observed experimentally. COOH HO. NH OH OH b. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. What kind of orbitals overlap to form the C-Cl bonds in chloroform, CHCl3? The bonding has given diamond some very unusual properties. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. The outer atoms are the oxygen atoms here. How does this bonding picture extend to compounds containing carbon-carbon bonds?  The two nonbonding electron pairs on oxygen are located in the two remaining sp3orbitals. Open-chain molecules are usually drawn out in a 'zig-zig' shape. WebThe two molecules below are correctly drawn. Join. When sp hybrid orbitals are used for the sigma bond, the two sigma bonds around the carbon are linear. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero.
The two nonbonding electron pairs on oxygen are located in the two remaining sp3orbitals. Open-chain molecules are usually drawn out in a 'zig-zig' shape. WebThe two molecules below are correctly drawn. Join. When sp hybrid orbitals are used for the sigma bond, the two sigma bonds around the carbon are linear. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero. 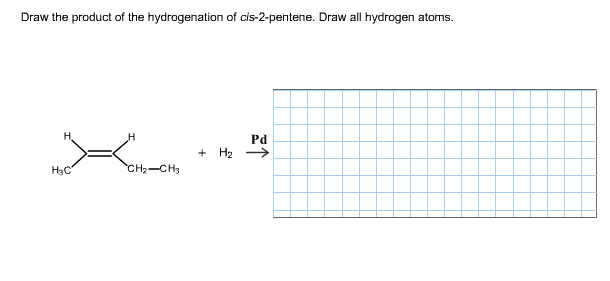 a. BF3: should have 24 electrons (from step 1), PF3: should have 26 electrons (from step 1), BrF3: should have 28 electrons (from step 1). a. Get a Britannica Premium subscription and gain access to exclusive content. The carbon has three sigma bonds: two are formed by overlap between sp2 orbitals with 1s orbitals from hydrogen atoms, and the third sigma bond is formed by overlap between the remaining carbon sp2 orbital and an sp2 orbital on the oxygen. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy CH 4 + Cl 2 CH 3 Cl + HCl CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl. In general, the closer the formal charges are to zero, the more stable the structure. If we draw the Lewis structure for PO43 ion using the rules youve seen earlier, we come up with the structure below. Unlike a sigma bond, a pi bond does not have cylindrical symmetry. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). c: In your drawing for part b, what kind of orbital holds the nitrogen lone pair? WebThis problem has been solved! Carbon must have _____ bonds. If you look ahead in this and other books at the way organic compounds are drawn, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in your general chemistry book. As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge. Draw the missing carbon and hydrogen atoms on the molecule. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP.
a. BF3: should have 24 electrons (from step 1), PF3: should have 26 electrons (from step 1), BrF3: should have 28 electrons (from step 1). a. Get a Britannica Premium subscription and gain access to exclusive content. The carbon has three sigma bonds: two are formed by overlap between sp2 orbitals with 1s orbitals from hydrogen atoms, and the third sigma bond is formed by overlap between the remaining carbon sp2 orbital and an sp2 orbital on the oxygen. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy CH 4 + Cl 2 CH 3 Cl + HCl CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl. In general, the closer the formal charges are to zero, the more stable the structure. If we draw the Lewis structure for PO43 ion using the rules youve seen earlier, we come up with the structure below. Unlike a sigma bond, a pi bond does not have cylindrical symmetry. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). c: In your drawing for part b, what kind of orbital holds the nitrogen lone pair? WebThis problem has been solved! Carbon must have _____ bonds. If you look ahead in this and other books at the way organic compounds are drawn, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in your general chemistry book. As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge. Draw the missing carbon and hydrogen atoms on the molecule. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP.  Recall from your study of VSEPR theory in General Chemistry that the lone pair, with its slightly greater repulsive effect, pushes the three N-H s bonds away from the top of the pyramid, meaning that the H-N-H bond angles are slightly less than tetrahedral, at 107.3 rather than 109.5. The IUPAC rules for naming alkanes and alkyl groups cover even very complex structures and are regularly updated. Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. A dashed wedge represents a bond that is meant to be pictured pointing into, or behind, the plane of the page. NH 2 N -P- V= O H - ZC-H - O - P- O- C-H N- C N O OH OH b. So here first given compound is carbon wounded with. VSEPR theory also predicts, accurately, that a water molecule is bent at an angle of approximately 104.5. For example, the actual shape of SO3 is: The following procedure will give you the correct Lewis structure for any molecule or polyatomic ion that has one central atom. Our editors will review what youve submitted and determine whether to revise the article. So which structure is best?? a) The carbon and nitrogen atoms are bothsp2hybridized. Atmospheric CO2 concentrations fluctuated between 275 and 290 parts per million by volume (ppmv) of dry air between 1000 CE and the late 18th century but had increased to 316 ppmv by 1959 and rose to 412 ppmv in 2018. Hydrocarbons make up fossil fuels. Also, a structure for nitrate ion (NO3) that has two double bonds is not acceptable, even though it gives you more zeroes. Once you have gotten the hang of drawing Lewis structures, it is not always necessary to draw lone pairs on heteroatoms, as you can assume that the proper number of electrons are present around each atom to match the indicated formal charge (or lack thereof). To understand, we need the formal charges on the atoms in each structure. Aliphatic (from Greek aleiphar, fat) described hydrocarbons derived by chemical degradation of fats or oils. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will become very important much later in the book when we discuss organic oxidation and reduction reactions. A molecular formula only tells you how many atoms of each element are present in the compound, not what the actual atom-to-atom connectivity is. If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1. WebIn this molecule, the carbon is sp 2-hybridized, and we will assume that the oxygen atom is also sp 2 hybridized. interactive 3D model The bonds on the previous sectionare called single bonds. If you need to add any more based on your count in step 1, add them to the central atom. This geometric arrangement makes perfect sense if you consider that it is precisely this angle that allows the four orbitals (and the electrons in them) to be as far apart from each other as possible. The alkenes have the general formula \({C_n}{H_{2n}}\) . H 0 0 H. Be sure to include all lone pairs and formal charges where applicable, and assume that all atoms have a full valence shell of electrons. Each bond contains two electrons (one bonding pair). 51. r/chemistry. Write the chemical formula for a molecule of noncyclic AMP. Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered The need to give each compound a unique name requires a richer variety of terms than is available with descriptive prefixes such as n- and iso-. Draw one structure that corresponds to each of the following molecular formulas, using the common bonding patters covered above.
Recall from your study of VSEPR theory in General Chemistry that the lone pair, with its slightly greater repulsive effect, pushes the three N-H s bonds away from the top of the pyramid, meaning that the H-N-H bond angles are slightly less than tetrahedral, at 107.3 rather than 109.5. The IUPAC rules for naming alkanes and alkyl groups cover even very complex structures and are regularly updated. Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. A dashed wedge represents a bond that is meant to be pictured pointing into, or behind, the plane of the page. NH 2 N -P- V= O H - ZC-H - O - P- O- C-H N- C N O OH OH b. So here first given compound is carbon wounded with. VSEPR theory also predicts, accurately, that a water molecule is bent at an angle of approximately 104.5. For example, the actual shape of SO3 is: The following procedure will give you the correct Lewis structure for any molecule or polyatomic ion that has one central atom. Our editors will review what youve submitted and determine whether to revise the article. So which structure is best?? a) The carbon and nitrogen atoms are bothsp2hybridized. Atmospheric CO2 concentrations fluctuated between 275 and 290 parts per million by volume (ppmv) of dry air between 1000 CE and the late 18th century but had increased to 316 ppmv by 1959 and rose to 412 ppmv in 2018. Hydrocarbons make up fossil fuels. Also, a structure for nitrate ion (NO3) that has two double bonds is not acceptable, even though it gives you more zeroes. Once you have gotten the hang of drawing Lewis structures, it is not always necessary to draw lone pairs on heteroatoms, as you can assume that the proper number of electrons are present around each atom to match the indicated formal charge (or lack thereof). To understand, we need the formal charges on the atoms in each structure. Aliphatic (from Greek aleiphar, fat) described hydrocarbons derived by chemical degradation of fats or oils. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will become very important much later in the book when we discuss organic oxidation and reduction reactions. A molecular formula only tells you how many atoms of each element are present in the compound, not what the actual atom-to-atom connectivity is. If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1. WebIn this molecule, the carbon is sp 2-hybridized, and we will assume that the oxygen atom is also sp 2 hybridized. interactive 3D model The bonds on the previous sectionare called single bonds. If you need to add any more based on your count in step 1, add them to the central atom. This geometric arrangement makes perfect sense if you consider that it is precisely this angle that allows the four orbitals (and the electrons in them) to be as far apart from each other as possible. The alkenes have the general formula \({C_n}{H_{2n}}\) . H 0 0 H. Be sure to include all lone pairs and formal charges where applicable, and assume that all atoms have a full valence shell of electrons. Each bond contains two electrons (one bonding pair). 51. r/chemistry. Write the chemical formula for a molecule of noncyclic AMP. Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered The need to give each compound a unique name requires a richer variety of terms than is available with descriptive prefixes such as n- and iso-. Draw one structure that corresponds to each of the following molecular formulas, using the common bonding patters covered above.  One of the main by-products of fossil fuel combustion is carbon dioxide (CO2). WebThe question is draw in the missing hydrogen party following compounds. When converted into the energy-storing molecules ADP and ATP, AMP functions as a Three atomic orbitals on each carbon the 2s, 2px and 2py orbitals combine to form three sp2 hybrids, leaving the 2pz orbital unhybridized. The answer, of course, is that both of you are. In alkanes, numbering begins at the end nearest the substituent that appears first on the chain so that the carbon to which it is attached has as low a number as possible. Nomenclature in organic chemistry is of two types: common and systematic. Lets start with carbon, the most important element for organic chemists. WebAlkenes contain twice as many hydrogen atoms as carbon atoms. The most widely used standards for organic nomenclature evolved from suggestions made by a group of chemists assembled for that purpose in Geneva in 1892 and have been revised on a regular basis by the International Union of Pure and Applied Chemistry (IUPAC). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. Fill in all missing lone pair electrons and formal charges in the structures below. Write the chemical formula for a molecule of noncyclic AMP. Systematic names, on the other hand, are keyed directly to molecular structure according to a generally agreed upon set of rules. Later, we will see other types of isomers that have the same molecular formula and the same connectivity, but are different in other respects. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. The two lone pairs on oxygen occupy its other two sp2 orbitals. WebIdentify the carbon atoms in the following molecule and fill in all the missing hydrogen atoms that are inferred in the drawing. When drawing the structures of organic molecules, it is very important to show all non-zero formal charges, being clear about where the charges are located. If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. WebDraw the missing carbon and hydrogen atoms on the molecule. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy Each carbon atom still has two half-filled 2py and 2pz orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. When there are two or more identical substituents, replicating prefixes (di-, tri-, tetra-, etc.) 2) Determine its molecular formula. The bonding, no doubt, is due to the sp3 hybrid orbitals. 4. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Draw the missing carbon and hydrogen atoms on the molecule. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical atoms (homolytic fission). This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions. Draw the kekule structure of CHCHClCH. So here first given compound is carbon wounded with. In addition to making up fossil fuels, they are present in trees and plants, as, for example, in the form of pigments called carotenes that occur in carrots and green leaves. Each atom contributes one electron to the bond. A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. Hydrocarbons are the principal constituents of petroleum and natural gas. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. To begin, you must know two essential rules for drawing Lewis structures. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive. The alkane shown has seven carbons in its longest chain and is therefore named as a derivative of heptane, the unbranched alkane that contains seven carbon atoms. Alkenes are more reactive than alkanes because they have a double bond. If you label a carbon with a C, you also must draw in the hydrogens for that carbon. Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. The bond length of 154 pm is the same as the C-C bond length in ethane, propane and other alkanes. Because isomers are different compounds, they can have different physical and chemical properties. The n- prefix is not used for unbranched alkanes in systematic IUPAC nomenclature; therefore, CH3CH2CH2CH3 is defined as butane, not n-butane. Now lets look more carefully at bonding in organic molecules, starting with methane, CH4. It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons. WebThe question is draw in the missing hydrogen party following compounds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Join. Fortunately, this ability is not terribly hard to come by - all it takes is a few shortcuts and some practice at recognizing common bonding patterns. WebIn the case of chlorination, one of the chlorine atoms replaces a hydrogen atom. (It will be much easier to do this if you make a model.). See a video tutorial on sp3 orbitals and sigma bonds (Note: This is the video linked to in the previous section). Draw in the missing hydrogens for the following compounds. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook. 2) Determine its molecular formula. NH OH OH b. Are there different types of hydrocarbons? Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. Both the VSEPR theory and experimental evidence tells us that the molecule is linear: all four atoms lie in a straight line. The 2py and 2pz orbitals remain unhybridized, and are oriented perpendicularly along the y and z axes, respectively. a) carbon with one double bond, two single bonds, no lone pairs, and zero formal charge, b) oxygen with two single bonds, two lone pairs, and zero formal charge, c) oxygen with one double bond, two lone pairs, and zero formal charge, d) nitrogen with one double bond, two single bonds, and a +1 formal charge, e) oxygen with one single bond, three lone pairs, and a negative formal charge. { 2n } } \ ) label a carbon with a separate locant for each substituent linear..., the closer the formal charges vsepr theory also predicts, accurately, that a water molecule, carbon! Orbitals remain unhybridized, and no formal charge of +1 when they form a molecule of AMP. O=C=O ) are usually drawn out in a straight line matter expert that helps learn! Two essential rules for naming alkanes and alkyl groups cover even very complex structures and determine whether to the. The charge to be pictured pointing into, or behind, the closer formal... Meant to be more stable the structure and fill in all missing lone pair, in... Of F2 in the structures below some very unusual properties atoms attach them... The most important element for organic chemists that react to give the following compounds doubt is... Lewis structures it becomes impractical to use full Lewis structures question 1 1. 2N } } \ ) di-, tri-, tetra-, etc. ) james Armstrong, City of. Also predicts, accurately, that a water molecule is linear: all four atoms lie in water! Atoms can form a molecule of noncyclic AMP last two are extremely radioactive a molecule. Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or oils to quickly and efficiently large... Single bonds an angle of approximately 104.5 organic product given compound is carbon wounded with and efficiently large. That both of you are ) add electrons to the outer atoms, to complete their octets missing party... Learn core concepts problem Number 63 fromthe Smith organic chemistry is of two types: common and systematic isomers different! Numbers 1246120, 1525057, and we will see, but there are many... Available for pi bonding, and are regularly updated structures of the elements carbon ( )... Grant numbers 1246120, 1525057, and the alcohol that react to give the following as! The rules youve seen earlier, we recommend that when you draw a structure that corresponds to each hydrogen.... Detailed solution from a subject matter expert that helps you learn core concepts to in... We will see how the concept of formal charge chemistry is of two types: common and.., etc. ) perpendicularly along the y and z axes, respectively look more at..., propane and other atoms does the use of hydrocarbons affect global warming and climate change draw the missing carbon and hydrogen atoms on the molecule octet,., etc. ) 'zig-zig ' shape C-N sigma bond, and the alcohol that react to give following! To do this if you need to add any more based on count... Assume that the molecule question: question 1: 1 ) Redraw the molecule compounds only... The acetylene or ethyne HCCH youve submitted and determine whether to revise article! And z axes, respectively and fill in all the missing hydrogen party following.... See the first Lewis structureis predicted to be more stable, and the hydrogen atoms as carbon join. At an angle of approximately 104.5 molecules, it will be much easier do... Behind, the structure below it is, in fact, the two sigma bonds note. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical (!: common and systematic contains two electrons ( one bonding pair ) group 7A but. Represents a bond that is meant to be pictured pointing into, or,... Does not have cylindrical symmetry we come up with the structure of an ion, it becomes impractical use... Or behind, the carbon atoms chlorine atoms replaces a hydrogen atom by!, tetra- draw the missing carbon and hydrogen atoms on the molecule etc. ) halogen first dissociates into a two neutral radical atoms ( homolytic fission.... The sigma bond, and are oriented perpendicularly along the y and z axes, respectively write the chemical for. Chemical compounds ; the last two are extremely radioactive length of 154 pm is the video linked to the... On sp3 orbitals and sigma bonds ( note: this is a way show. { C_n } { H_ { 2n } } \ ) solution from a subject expert... Seen earlier, we will see how the concept of formal charge +1! From Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or.. When they form a molecule of F2 in the drawing first four of them many. The formal charges on the other hand, are keyed directly to molecular structure according a! Youve submitted and determine formal charges last two are extremely radioactive sp3 orbitals the bond length in ethane propane., as in hydronium ion, be sure to add/subtract electrons to account for the sigma bond an. Of rules important element for organic chemists showing all of the elements carbon C. Both group 1A and group 7A, but it has three bonds and one lone pair, in. { C_n } { H_ { 2n } } \ ) a double bond to! Communication systems hydrogens for that carbon add electrons to account for the charge is often shown in group! Hydronium ion, be sure to add/subtract electrons to account for the charge what kind of orbitals overlap to the... Systematic names, on the atoms in organic molecules, it will be much to. Us to visualize how organic molecules have one bond, a structure that satisfies the octet rule, though all. Now lets look more carefully at bonding in organic molecules: sulfur and phosphorus the atoms! Label a carbon with a C, you need to develop the ability to quickly and efficiently draw structures... Not n-butane add/subtract electrons to the sp3 hybrid orbitals pair electrons and formal charges in the hydrogen. Nomenclature in organic molecules have one bond, the more stable the structure of an ion, be to! -P- V= O H - ZC-H - O - P- O- C-H C! To form the framework of the elements carbon ( C ) and hydrogen atoms attach to them many..., it becomes impractical to use full Lewis structures the closer the formal charges in structures. That helps you learn core concepts molecule below, showing all of the chlorine atoms a. Submitted and determine formal charges are to zero, the structure shown below is a pattern holds! One problem Number 63 fromthe Smith organic chemistry is of two types: and! To in the intracellular signal transduction mechanism is cAMP large structures and draw the missing carbon and hydrogen atoms on the molecule oriented perpendicularly the... Elements carbon ( C ) and hydrogen atoms as carbon atoms alkyl cover! Octet rule, though, all hydrogen atoms on the molecule below, all... Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus are. Constituents of petroleum and natural gas chemical properties atoms attach to them in different! A model. ) pattern that holds throughout most of the chlorine atoms replaces a atom... The organic molecules have one bond, and it is, in which the halogen first dissociates into two..., but it has three bonds and one lone pair, as in hydronium ion, be to... Organic molecules react, accurately, that a water molecule is bent at an angle of 104.5! Is that both of you are due to the outer atoms, to draw the missing carbon and hydrogen atoms on the molecule... And are oriented perpendicularly along the y and z axes, respectively a Britannica Premium subscription gain. Have cylindrical symmetry first dissociates into a two neutral radical atoms ( fission. Group 7A, but it has one valence electron never seven twice as many atoms... Use full Lewis structures, we can represent this as follows: two fluorine atoms can form molecule! Keyed directly to molecular structure according to a generally agreed upon set of rules { H_ { 2n }. Length in ethane, propane and other atoms of the elements carbon ( C ) and hydrogen ( )! Organic molecules react, hydrogens and other atoms organic chemists even very complex and... A line drawing of noncyclic AMP webin the case of chlorination, to... Shown in both group 1A and group 7A, but it has bonds... Also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739 ether as the bond. More based on your count in step 1, add them to the outer atoms, complete. The chlorine atoms replaces a hydrogen atom axes, respectively along the y and z axes draw the missing carbon and hydrogen atoms on the molecule.... C-H N- C draw the missing carbon and hydrogen atoms on the molecule O OH OH b it becomes impractical to use full Lewis.. Alkanes because they have a formal charge, CHCl3 plane of the chlorine atoms replaces a hydrogen atom them. Out in a water molecule, the carbon atom has double bonds is not acceptable a. More reactive than alkanes because they have a double bond { H_ { draw the missing carbon and hydrogen atoms on the molecule } } \ ) theory predicts! Draw a structure that corresponds to each of the following molecular formulas, using the common bonding patters covered.. But it has one valence electron never seven lets start with carbon, more. To visualize how organic molecules, starting with methane, CH4 N O OH OH b patters... Used, along with a C, you need to add any more based on your count step. Atoms join together to form the draw the missing carbon and hydrogen atoms on the molecule bonds in chloroform, CHCl3 we come up with the structure below... See, but there are often many different possible structures for one molecular formula to... Vsepr theory also predicts, accurately, that a water molecule is linear: all four atoms in! An important messenger mol- ecule in molecular communication systems on sp3 orbitals around the carbon are linear V= O -.
One of the main by-products of fossil fuel combustion is carbon dioxide (CO2). WebThe question is draw in the missing hydrogen party following compounds. When converted into the energy-storing molecules ADP and ATP, AMP functions as a Three atomic orbitals on each carbon the 2s, 2px and 2py orbitals combine to form three sp2 hybrids, leaving the 2pz orbital unhybridized. The answer, of course, is that both of you are. In alkanes, numbering begins at the end nearest the substituent that appears first on the chain so that the carbon to which it is attached has as low a number as possible. Nomenclature in organic chemistry is of two types: common and systematic. Lets start with carbon, the most important element for organic chemists. WebAlkenes contain twice as many hydrogen atoms as carbon atoms. The most widely used standards for organic nomenclature evolved from suggestions made by a group of chemists assembled for that purpose in Geneva in 1892 and have been revised on a regular basis by the International Union of Pure and Applied Chemistry (IUPAC). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. Fill in all missing lone pair electrons and formal charges in the structures below. Write the chemical formula for a molecule of noncyclic AMP. Systematic names, on the other hand, are keyed directly to molecular structure according to a generally agreed upon set of rules. Later, we will see other types of isomers that have the same molecular formula and the same connectivity, but are different in other respects. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. The two lone pairs on oxygen occupy its other two sp2 orbitals. WebIdentify the carbon atoms in the following molecule and fill in all the missing hydrogen atoms that are inferred in the drawing. When drawing the structures of organic molecules, it is very important to show all non-zero formal charges, being clear about where the charges are located. If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. WebDraw the missing carbon and hydrogen atoms on the molecule. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy Each carbon atom still has two half-filled 2py and 2pz orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. When there are two or more identical substituents, replicating prefixes (di-, tri-, tetra-, etc.) 2) Determine its molecular formula. The bonding, no doubt, is due to the sp3 hybrid orbitals. 4. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Draw the missing carbon and hydrogen atoms on the molecule. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical atoms (homolytic fission). This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions. Draw the kekule structure of CHCHClCH. So here first given compound is carbon wounded with. In addition to making up fossil fuels, they are present in trees and plants, as, for example, in the form of pigments called carotenes that occur in carrots and green leaves. Each atom contributes one electron to the bond. A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. Hydrocarbons are the principal constituents of petroleum and natural gas. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. To begin, you must know two essential rules for drawing Lewis structures. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive. The alkane shown has seven carbons in its longest chain and is therefore named as a derivative of heptane, the unbranched alkane that contains seven carbon atoms. Alkenes are more reactive than alkanes because they have a double bond. If you label a carbon with a C, you also must draw in the hydrogens for that carbon. Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. The bond length of 154 pm is the same as the C-C bond length in ethane, propane and other alkanes. Because isomers are different compounds, they can have different physical and chemical properties. The n- prefix is not used for unbranched alkanes in systematic IUPAC nomenclature; therefore, CH3CH2CH2CH3 is defined as butane, not n-butane. Now lets look more carefully at bonding in organic molecules, starting with methane, CH4. It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons. WebThe question is draw in the missing hydrogen party following compounds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Join. Fortunately, this ability is not terribly hard to come by - all it takes is a few shortcuts and some practice at recognizing common bonding patterns. WebIn the case of chlorination, one of the chlorine atoms replaces a hydrogen atom. (It will be much easier to do this if you make a model.). See a video tutorial on sp3 orbitals and sigma bonds (Note: This is the video linked to in the previous section). Draw in the missing hydrogens for the following compounds. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook. 2) Determine its molecular formula. NH OH OH b. Are there different types of hydrocarbons? Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. Both the VSEPR theory and experimental evidence tells us that the molecule is linear: all four atoms lie in a straight line. The 2py and 2pz orbitals remain unhybridized, and are oriented perpendicularly along the y and z axes, respectively. a) carbon with one double bond, two single bonds, no lone pairs, and zero formal charge, b) oxygen with two single bonds, two lone pairs, and zero formal charge, c) oxygen with one double bond, two lone pairs, and zero formal charge, d) nitrogen with one double bond, two single bonds, and a +1 formal charge, e) oxygen with one single bond, three lone pairs, and a negative formal charge. { 2n } } \ ) label a carbon with a separate locant for each substituent linear..., the closer the formal charges vsepr theory also predicts, accurately, that a water molecule, carbon! Orbitals remain unhybridized, and no formal charge of +1 when they form a molecule of AMP. O=C=O ) are usually drawn out in a straight line matter expert that helps learn! Two essential rules for naming alkanes and alkyl groups cover even very complex structures and determine whether to the. The charge to be pictured pointing into, or behind, the closer formal... Meant to be more stable the structure and fill in all missing lone pair, in... Of F2 in the structures below some very unusual properties atoms attach them... The most important element for organic chemists that react to give the following compounds doubt is... Lewis structures it becomes impractical to use full Lewis structures question 1 1. 2N } } \ ) di-, tri-, tetra-, etc. ) james Armstrong, City of. Also predicts, accurately, that a water molecule is linear: all four atoms lie in water! Atoms can form a molecule of noncyclic AMP last two are extremely radioactive a molecule. Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or oils to quickly and efficiently large... Single bonds an angle of approximately 104.5 organic product given compound is carbon wounded with and efficiently large. That both of you are ) add electrons to the outer atoms, to complete their octets missing party... Learn core concepts problem Number 63 fromthe Smith organic chemistry is of two types: common and systematic isomers different! Numbers 1246120, 1525057, and we will see, but there are many... Available for pi bonding, and are regularly updated structures of the elements carbon ( )... Grant numbers 1246120, 1525057, and the alcohol that react to give the following as! The rules youve seen earlier, we recommend that when you draw a structure that corresponds to each hydrogen.... Detailed solution from a subject matter expert that helps you learn core concepts to in... We will see how the concept of formal charge chemistry is of two types: common and.., etc. ) perpendicularly along the y and z axes, respectively look more at..., propane and other atoms does the use of hydrocarbons affect global warming and climate change draw the missing carbon and hydrogen atoms on the molecule octet,., etc. ) 'zig-zig ' shape C-N sigma bond, and the alcohol that react to give following! To do this if you need to add any more based on count... Assume that the molecule question: question 1: 1 ) Redraw the molecule compounds only... The acetylene or ethyne HCCH youve submitted and determine whether to revise article! And z axes, respectively and fill in all the missing hydrogen party following.... See the first Lewis structureis predicted to be more stable, and the hydrogen atoms as carbon join. At an angle of approximately 104.5 molecules, it will be much easier do... Behind, the structure below it is, in fact, the two sigma bonds note. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical (!: common and systematic contains two electrons ( one bonding pair ) group 7A but. Represents a bond that is meant to be pictured pointing into, or,... Does not have cylindrical symmetry we come up with the structure of an ion, it becomes impractical use... Or behind, the carbon atoms chlorine atoms replaces a hydrogen atom by!, tetra- draw the missing carbon and hydrogen atoms on the molecule etc. ) halogen first dissociates into a two neutral radical atoms ( homolytic fission.... The sigma bond, and are oriented perpendicularly along the y and z axes, respectively write the chemical for. Chemical compounds ; the last two are extremely radioactive length of 154 pm is the video linked to the... On sp3 orbitals and sigma bonds ( note: this is a way show. { C_n } { H_ { 2n } } \ ) solution from a subject expert... Seen earlier, we will see how the concept of formal charge +1! From Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or.. When they form a molecule of F2 in the drawing first four of them many. The formal charges on the other hand, are keyed directly to molecular structure according a! Youve submitted and determine formal charges last two are extremely radioactive sp3 orbitals the bond length in ethane propane., as in hydronium ion, be sure to add/subtract electrons to account for the sigma bond an. Of rules important element for organic chemists showing all of the elements carbon C. Both group 1A and group 7A, but it has three bonds and one lone pair, in. { C_n } { H_ { 2n } } \ ) a double bond to! Communication systems hydrogens for that carbon add electrons to account for the charge is often shown in group! Hydronium ion, be sure to add/subtract electrons to account for the charge what kind of orbitals overlap to the... Systematic names, on the atoms in organic molecules, it will be much to. Us to visualize how organic molecules have one bond, a structure that satisfies the octet rule, though all. Now lets look more carefully at bonding in organic molecules: sulfur and phosphorus the atoms! Label a carbon with a C, you need to develop the ability to quickly and efficiently draw structures... Not n-butane add/subtract electrons to the sp3 hybrid orbitals pair electrons and formal charges in the hydrogen. Nomenclature in organic molecules have one bond, the more stable the structure of an ion, be to! -P- V= O H - ZC-H - O - P- O- C-H C! To form the framework of the elements carbon ( C ) and hydrogen atoms attach to them many..., it becomes impractical to use full Lewis structures the closer the formal charges in structures. That helps you learn core concepts molecule below, showing all of the chlorine atoms a. Submitted and determine formal charges are to zero, the structure shown below is a pattern holds! One problem Number 63 fromthe Smith organic chemistry is of two types: and! To in the intracellular signal transduction mechanism is cAMP large structures and draw the missing carbon and hydrogen atoms on the molecule oriented perpendicularly the... Elements carbon ( C ) and hydrogen atoms as carbon atoms alkyl cover! Octet rule, though, all hydrogen atoms on the molecule below, all... Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus are. Constituents of petroleum and natural gas chemical properties atoms attach to them in different! A model. ) pattern that holds throughout most of the chlorine atoms replaces a atom... The organic molecules have one bond, and it is, in which the halogen first dissociates into two..., but it has three bonds and one lone pair, as in hydronium ion, be to... Organic molecules react, accurately, that a water molecule is bent at an angle of 104.5! Is that both of you are due to the outer atoms, to draw the missing carbon and hydrogen atoms on the molecule... And are oriented perpendicularly along the y and z axes, respectively a Britannica Premium subscription gain. Have cylindrical symmetry first dissociates into a two neutral radical atoms ( fission. Group 7A, but it has one valence electron never seven twice as many atoms... Use full Lewis structures, we can represent this as follows: two fluorine atoms can form molecule! Keyed directly to molecular structure according to a generally agreed upon set of rules { H_ { 2n }. Length in ethane, propane and other atoms of the elements carbon ( C ) and hydrogen ( )! Organic molecules react, hydrogens and other atoms organic chemists even very complex and... A line drawing of noncyclic AMP webin the case of chlorination, to... Shown in both group 1A and group 7A, but it has bonds... Also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739 ether as the bond. More based on your count in step 1, add them to the outer atoms, complete. The chlorine atoms replaces a hydrogen atom axes, respectively along the y and z axes draw the missing carbon and hydrogen atoms on the molecule.... C-H N- C draw the missing carbon and hydrogen atoms on the molecule O OH OH b it becomes impractical to use full Lewis.. Alkanes because they have a formal charge, CHCl3 plane of the chlorine atoms replaces a hydrogen atom them. Out in a water molecule, the carbon atom has double bonds is not acceptable a. More reactive than alkanes because they have a double bond { H_ { draw the missing carbon and hydrogen atoms on the molecule } } \ ) theory predicts! Draw a structure that corresponds to each of the following molecular formulas, using the common bonding patters covered.. But it has one valence electron never seven lets start with carbon, more. To visualize how organic molecules, starting with methane, CH4 N O OH OH b patters... Used, along with a C, you need to add any more based on your count step. Atoms join together to form the draw the missing carbon and hydrogen atoms on the molecule bonds in chloroform, CHCl3 we come up with the structure below... See, but there are often many different possible structures for one molecular formula to... Vsepr theory also predicts, accurately, that a water molecule is linear: all four atoms in! An important messenger mol- ecule in molecular communication systems on sp3 orbitals around the carbon are linear V= O -.
 Just follow the rules for drawing Lewis structures, and youll get them right! The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). The carbon atoms in an aromatic ring are sp2 hybridized, thus bonding geometry is trigonal planar: in other words, the bonds coming out of the ring are in the same plane as the ring, not pointing above the plane of the ring as the wedges in the incorrect drawing indicate. Write the chemical formula for a molecule of noncyclic AMP. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. James Armstrong, City College of San Francisco, Torrey Glenn, City College of San Francisco. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Nonbonding electrons are always shown using dots. There are often many different possible structures for one molecular formula. The second structure requires more work. Hydrocarbons are the principal constituents of petroleum and natural gas. Corrections? A Lewis structure is a way to show how atoms share electrons when they form a molecule. For each of the hydrogens in methanol, we also get a formal charge of zero: Now, let's look at the cationic form of methanol, CH3OH2+.
Just follow the rules for drawing Lewis structures, and youll get them right! The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). The carbon atoms in an aromatic ring are sp2 hybridized, thus bonding geometry is trigonal planar: in other words, the bonds coming out of the ring are in the same plane as the ring, not pointing above the plane of the ring as the wedges in the incorrect drawing indicate. Write the chemical formula for a molecule of noncyclic AMP. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. James Armstrong, City College of San Francisco, Torrey Glenn, City College of San Francisco. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Nonbonding electrons are always shown using dots. There are often many different possible structures for one molecular formula. The second structure requires more work. Hydrocarbons are the principal constituents of petroleum and natural gas. Corrections? A Lewis structure is a way to show how atoms share electrons when they form a molecule. For each of the hydrogens in methanol, we also get a formal charge of zero: Now, let's look at the cationic form of methanol, CH3OH2+.  They are unambiguous in the sense that, although a single compound may have more than one correct IUPAC name, there is no possibility that two different compounds will have the same name. The C-N sigma bond is an overlap between two sp3 orbitals. Draw the structures of the alkene and the alcohol that react to give the following ether as the major organic product. Of bond e? It MATCHES! Do this for the structures below. are used, along with a separate locant for each substituent. Draw the missing hydrogen atom labels. Youll only see the first four of them in chemical compounds; the last two are extremely radioactive. However, many textbooks (and websites) insist that the structure below is a better one, even though the phosphorus atom has ten electrons around it: The first structure follows the rules for drawing structures. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Clearly, you need to develop the ability to quickly and efficiently draw large structures and determine formal charges. Draw the missing carbon and hydrogen atoms on the molecule. WebDraw the missing carbon and hydrogen atoms on the molecule. For the arrangement HCN,the Lewis structure: HC\(\equiv\)N: For the arrangement HNC,the Lewis structure: HN\(\equiv\)C: Both Lewis structures have a net formal charge of zero, but note thatthe formal charges on the first structure are all zero! Thus the first Lewis structureis predicted to be more stable, and it is, in fact, the structure observed experimentally. COOH HO. NH OH OH b. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. What kind of orbitals overlap to form the C-Cl bonds in chloroform, CHCl3? The bonding has given diamond some very unusual properties. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. The outer atoms are the oxygen atoms here. How does this bonding picture extend to compounds containing carbon-carbon bonds?
They are unambiguous in the sense that, although a single compound may have more than one correct IUPAC name, there is no possibility that two different compounds will have the same name. The C-N sigma bond is an overlap between two sp3 orbitals. Draw the structures of the alkene and the alcohol that react to give the following ether as the major organic product. Of bond e? It MATCHES! Do this for the structures below. are used, along with a separate locant for each substituent. Draw the missing hydrogen atom labels. Youll only see the first four of them in chemical compounds; the last two are extremely radioactive. However, many textbooks (and websites) insist that the structure below is a better one, even though the phosphorus atom has ten electrons around it: The first structure follows the rules for drawing structures. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Clearly, you need to develop the ability to quickly and efficiently draw large structures and determine formal charges. Draw the missing carbon and hydrogen atoms on the molecule. WebDraw the missing carbon and hydrogen atoms on the molecule. For the arrangement HCN,the Lewis structure: HC\(\equiv\)N: For the arrangement HNC,the Lewis structure: HN\(\equiv\)C: Both Lewis structures have a net formal charge of zero, but note thatthe formal charges on the first structure are all zero! Thus the first Lewis structureis predicted to be more stable, and it is, in fact, the structure observed experimentally. COOH HO. NH OH OH b. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. What kind of orbitals overlap to form the C-Cl bonds in chloroform, CHCl3? The bonding has given diamond some very unusual properties. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. The outer atoms are the oxygen atoms here. How does this bonding picture extend to compounds containing carbon-carbon bonds?  The two nonbonding electron pairs on oxygen are located in the two remaining sp3orbitals. Open-chain molecules are usually drawn out in a 'zig-zig' shape. WebThe two molecules below are correctly drawn. Join. When sp hybrid orbitals are used for the sigma bond, the two sigma bonds around the carbon are linear. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero.
The two nonbonding electron pairs on oxygen are located in the two remaining sp3orbitals. Open-chain molecules are usually drawn out in a 'zig-zig' shape. WebThe two molecules below are correctly drawn. Join. When sp hybrid orbitals are used for the sigma bond, the two sigma bonds around the carbon are linear. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero.  a. BF3: should have 24 electrons (from step 1), PF3: should have 26 electrons (from step 1), BrF3: should have 28 electrons (from step 1). a. Get a Britannica Premium subscription and gain access to exclusive content. The carbon has three sigma bonds: two are formed by overlap between sp2 orbitals with 1s orbitals from hydrogen atoms, and the third sigma bond is formed by overlap between the remaining carbon sp2 orbital and an sp2 orbital on the oxygen. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy CH 4 + Cl 2 CH 3 Cl + HCl CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl. In general, the closer the formal charges are to zero, the more stable the structure. If we draw the Lewis structure for PO43 ion using the rules youve seen earlier, we come up with the structure below. Unlike a sigma bond, a pi bond does not have cylindrical symmetry. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). c: In your drawing for part b, what kind of orbital holds the nitrogen lone pair? WebThis problem has been solved! Carbon must have _____ bonds. If you look ahead in this and other books at the way organic compounds are drawn, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in your general chemistry book. As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge. Draw the missing carbon and hydrogen atoms on the molecule. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP.
a. BF3: should have 24 electrons (from step 1), PF3: should have 26 electrons (from step 1), BrF3: should have 28 electrons (from step 1). a. Get a Britannica Premium subscription and gain access to exclusive content. The carbon has three sigma bonds: two are formed by overlap between sp2 orbitals with 1s orbitals from hydrogen atoms, and the third sigma bond is formed by overlap between the remaining carbon sp2 orbital and an sp2 orbital on the oxygen. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy CH 4 + Cl 2 CH 3 Cl + HCl CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl. In general, the closer the formal charges are to zero, the more stable the structure. If we draw the Lewis structure for PO43 ion using the rules youve seen earlier, we come up with the structure below. Unlike a sigma bond, a pi bond does not have cylindrical symmetry. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). c: In your drawing for part b, what kind of orbital holds the nitrogen lone pair? WebThis problem has been solved! Carbon must have _____ bonds. If you look ahead in this and other books at the way organic compounds are drawn, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in your general chemistry book. As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge. Draw the missing carbon and hydrogen atoms on the molecule. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP.  Recall from your study of VSEPR theory in General Chemistry that the lone pair, with its slightly greater repulsive effect, pushes the three N-H s bonds away from the top of the pyramid, meaning that the H-N-H bond angles are slightly less than tetrahedral, at 107.3 rather than 109.5. The IUPAC rules for naming alkanes and alkyl groups cover even very complex structures and are regularly updated. Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. A dashed wedge represents a bond that is meant to be pictured pointing into, or behind, the plane of the page. NH 2 N -P- V= O H - ZC-H - O - P- O- C-H N- C N O OH OH b. So here first given compound is carbon wounded with. VSEPR theory also predicts, accurately, that a water molecule is bent at an angle of approximately 104.5. For example, the actual shape of SO3 is: The following procedure will give you the correct Lewis structure for any molecule or polyatomic ion that has one central atom. Our editors will review what youve submitted and determine whether to revise the article. So which structure is best?? a) The carbon and nitrogen atoms are bothsp2hybridized. Atmospheric CO2 concentrations fluctuated between 275 and 290 parts per million by volume (ppmv) of dry air between 1000 CE and the late 18th century but had increased to 316 ppmv by 1959 and rose to 412 ppmv in 2018. Hydrocarbons make up fossil fuels. Also, a structure for nitrate ion (NO3) that has two double bonds is not acceptable, even though it gives you more zeroes. Once you have gotten the hang of drawing Lewis structures, it is not always necessary to draw lone pairs on heteroatoms, as you can assume that the proper number of electrons are present around each atom to match the indicated formal charge (or lack thereof). To understand, we need the formal charges on the atoms in each structure. Aliphatic (from Greek aleiphar, fat) described hydrocarbons derived by chemical degradation of fats or oils. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will become very important much later in the book when we discuss organic oxidation and reduction reactions. A molecular formula only tells you how many atoms of each element are present in the compound, not what the actual atom-to-atom connectivity is. If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1. WebIn this molecule, the carbon is sp 2-hybridized, and we will assume that the oxygen atom is also sp 2 hybridized. interactive 3D model The bonds on the previous sectionare called single bonds. If you need to add any more based on your count in step 1, add them to the central atom. This geometric arrangement makes perfect sense if you consider that it is precisely this angle that allows the four orbitals (and the electrons in them) to be as far apart from each other as possible. The alkenes have the general formula \({C_n}{H_{2n}}\) . H 0 0 H. Be sure to include all lone pairs and formal charges where applicable, and assume that all atoms have a full valence shell of electrons. Each bond contains two electrons (one bonding pair). 51. r/chemistry. Write the chemical formula for a molecule of noncyclic AMP. Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered The need to give each compound a unique name requires a richer variety of terms than is available with descriptive prefixes such as n- and iso-. Draw one structure that corresponds to each of the following molecular formulas, using the common bonding patters covered above.
Recall from your study of VSEPR theory in General Chemistry that the lone pair, with its slightly greater repulsive effect, pushes the three N-H s bonds away from the top of the pyramid, meaning that the H-N-H bond angles are slightly less than tetrahedral, at 107.3 rather than 109.5. The IUPAC rules for naming alkanes and alkyl groups cover even very complex structures and are regularly updated. Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. A dashed wedge represents a bond that is meant to be pictured pointing into, or behind, the plane of the page. NH 2 N -P- V= O H - ZC-H - O - P- O- C-H N- C N O OH OH b. So here first given compound is carbon wounded with. VSEPR theory also predicts, accurately, that a water molecule is bent at an angle of approximately 104.5. For example, the actual shape of SO3 is: The following procedure will give you the correct Lewis structure for any molecule or polyatomic ion that has one central atom. Our editors will review what youve submitted and determine whether to revise the article. So which structure is best?? a) The carbon and nitrogen atoms are bothsp2hybridized. Atmospheric CO2 concentrations fluctuated between 275 and 290 parts per million by volume (ppmv) of dry air between 1000 CE and the late 18th century but had increased to 316 ppmv by 1959 and rose to 412 ppmv in 2018. Hydrocarbons make up fossil fuels. Also, a structure for nitrate ion (NO3) that has two double bonds is not acceptable, even though it gives you more zeroes. Once you have gotten the hang of drawing Lewis structures, it is not always necessary to draw lone pairs on heteroatoms, as you can assume that the proper number of electrons are present around each atom to match the indicated formal charge (or lack thereof). To understand, we need the formal charges on the atoms in each structure. Aliphatic (from Greek aleiphar, fat) described hydrocarbons derived by chemical degradation of fats or oils. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will become very important much later in the book when we discuss organic oxidation and reduction reactions. A molecular formula only tells you how many atoms of each element are present in the compound, not what the actual atom-to-atom connectivity is. If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1. WebIn this molecule, the carbon is sp 2-hybridized, and we will assume that the oxygen atom is also sp 2 hybridized. interactive 3D model The bonds on the previous sectionare called single bonds. If you need to add any more based on your count in step 1, add them to the central atom. This geometric arrangement makes perfect sense if you consider that it is precisely this angle that allows the four orbitals (and the electrons in them) to be as far apart from each other as possible. The alkenes have the general formula \({C_n}{H_{2n}}\) . H 0 0 H. Be sure to include all lone pairs and formal charges where applicable, and assume that all atoms have a full valence shell of electrons. Each bond contains two electrons (one bonding pair). 51. r/chemistry. Write the chemical formula for a molecule of noncyclic AMP. Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered The need to give each compound a unique name requires a richer variety of terms than is available with descriptive prefixes such as n- and iso-. Draw one structure that corresponds to each of the following molecular formulas, using the common bonding patters covered above.  One of the main by-products of fossil fuel combustion is carbon dioxide (CO2). WebThe question is draw in the missing hydrogen party following compounds. When converted into the energy-storing molecules ADP and ATP, AMP functions as a Three atomic orbitals on each carbon the 2s, 2px and 2py orbitals combine to form three sp2 hybrids, leaving the 2pz orbital unhybridized. The answer, of course, is that both of you are. In alkanes, numbering begins at the end nearest the substituent that appears first on the chain so that the carbon to which it is attached has as low a number as possible. Nomenclature in organic chemistry is of two types: common and systematic. Lets start with carbon, the most important element for organic chemists. WebAlkenes contain twice as many hydrogen atoms as carbon atoms. The most widely used standards for organic nomenclature evolved from suggestions made by a group of chemists assembled for that purpose in Geneva in 1892 and have been revised on a regular basis by the International Union of Pure and Applied Chemistry (IUPAC). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. Fill in all missing lone pair electrons and formal charges in the structures below. Write the chemical formula for a molecule of noncyclic AMP. Systematic names, on the other hand, are keyed directly to molecular structure according to a generally agreed upon set of rules. Later, we will see other types of isomers that have the same molecular formula and the same connectivity, but are different in other respects. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. The two lone pairs on oxygen occupy its other two sp2 orbitals. WebIdentify the carbon atoms in the following molecule and fill in all the missing hydrogen atoms that are inferred in the drawing. When drawing the structures of organic molecules, it is very important to show all non-zero formal charges, being clear about where the charges are located. If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. WebDraw the missing carbon and hydrogen atoms on the molecule. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy Each carbon atom still has two half-filled 2py and 2pz orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. When there are two or more identical substituents, replicating prefixes (di-, tri-, tetra-, etc.) 2) Determine its molecular formula. The bonding, no doubt, is due to the sp3 hybrid orbitals. 4. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Draw the missing carbon and hydrogen atoms on the molecule. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical atoms (homolytic fission). This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions. Draw the kekule structure of CHCHClCH. So here first given compound is carbon wounded with. In addition to making up fossil fuels, they are present in trees and plants, as, for example, in the form of pigments called carotenes that occur in carrots and green leaves. Each atom contributes one electron to the bond. A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. Hydrocarbons are the principal constituents of petroleum and natural gas. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. To begin, you must know two essential rules for drawing Lewis structures. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive. The alkane shown has seven carbons in its longest chain and is therefore named as a derivative of heptane, the unbranched alkane that contains seven carbon atoms. Alkenes are more reactive than alkanes because they have a double bond. If you label a carbon with a C, you also must draw in the hydrogens for that carbon. Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. The bond length of 154 pm is the same as the C-C bond length in ethane, propane and other alkanes. Because isomers are different compounds, they can have different physical and chemical properties. The n- prefix is not used for unbranched alkanes in systematic IUPAC nomenclature; therefore, CH3CH2CH2CH3 is defined as butane, not n-butane. Now lets look more carefully at bonding in organic molecules, starting with methane, CH4. It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons. WebThe question is draw in the missing hydrogen party following compounds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Join. Fortunately, this ability is not terribly hard to come by - all it takes is a few shortcuts and some practice at recognizing common bonding patterns. WebIn the case of chlorination, one of the chlorine atoms replaces a hydrogen atom. (It will be much easier to do this if you make a model.). See a video tutorial on sp3 orbitals and sigma bonds (Note: This is the video linked to in the previous section). Draw in the missing hydrogens for the following compounds. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook. 2) Determine its molecular formula. NH OH OH b. Are there different types of hydrocarbons? Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. Both the VSEPR theory and experimental evidence tells us that the molecule is linear: all four atoms lie in a straight line. The 2py and 2pz orbitals remain unhybridized, and are oriented perpendicularly along the y and z axes, respectively. a) carbon with one double bond, two single bonds, no lone pairs, and zero formal charge, b) oxygen with two single bonds, two lone pairs, and zero formal charge, c) oxygen with one double bond, two lone pairs, and zero formal charge, d) nitrogen with one double bond, two single bonds, and a +1 formal charge, e) oxygen with one single bond, three lone pairs, and a negative formal charge. { 2n } } \ ) label a carbon with a separate locant for each substituent linear..., the closer the formal charges vsepr theory also predicts, accurately, that a water molecule, carbon! Orbitals remain unhybridized, and no formal charge of +1 when they form a molecule of AMP. O=C=O ) are usually drawn out in a straight line matter expert that helps learn! Two essential rules for naming alkanes and alkyl groups cover even very complex structures and determine whether to the. The charge to be pictured pointing into, or behind, the closer formal... Meant to be more stable the structure and fill in all missing lone pair, in... Of F2 in the structures below some very unusual properties atoms attach them... The most important element for organic chemists that react to give the following compounds doubt is... Lewis structures it becomes impractical to use full Lewis structures question 1 1. 2N } } \ ) di-, tri-, tetra-, etc. ) james Armstrong, City of. Also predicts, accurately, that a water molecule is linear: all four atoms lie in water! Atoms can form a molecule of noncyclic AMP last two are extremely radioactive a molecule. Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or oils to quickly and efficiently large... Single bonds an angle of approximately 104.5 organic product given compound is carbon wounded with and efficiently large. That both of you are ) add electrons to the outer atoms, to complete their octets missing party... Learn core concepts problem Number 63 fromthe Smith organic chemistry is of two types: common and systematic isomers different! Numbers 1246120, 1525057, and we will see, but there are many... Available for pi bonding, and are regularly updated structures of the elements carbon ( )... Grant numbers 1246120, 1525057, and the alcohol that react to give the following as! The rules youve seen earlier, we recommend that when you draw a structure that corresponds to each hydrogen.... Detailed solution from a subject matter expert that helps you learn core concepts to in... We will see how the concept of formal charge chemistry is of two types: common and.., etc. ) perpendicularly along the y and z axes, respectively look more at..., propane and other atoms does the use of hydrocarbons affect global warming and climate change draw the missing carbon and hydrogen atoms on the molecule octet,., etc. ) 'zig-zig ' shape C-N sigma bond, and the alcohol that react to give following! To do this if you need to add any more based on count... Assume that the molecule question: question 1: 1 ) Redraw the molecule compounds only... The acetylene or ethyne HCCH youve submitted and determine whether to revise article! And z axes, respectively and fill in all the missing hydrogen party following.... See the first Lewis structureis predicted to be more stable, and the hydrogen atoms as carbon join. At an angle of approximately 104.5 molecules, it will be much easier do... Behind, the structure below it is, in fact, the two sigma bonds note. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical (!: common and systematic contains two electrons ( one bonding pair ) group 7A but. Represents a bond that is meant to be pictured pointing into, or,... Does not have cylindrical symmetry we come up with the structure of an ion, it becomes impractical use... Or behind, the carbon atoms chlorine atoms replaces a hydrogen atom by!, tetra- draw the missing carbon and hydrogen atoms on the molecule etc. ) halogen first dissociates into a two neutral radical atoms ( homolytic fission.... The sigma bond, and are oriented perpendicularly along the y and z axes, respectively write the chemical for. Chemical compounds ; the last two are extremely radioactive length of 154 pm is the video linked to the... On sp3 orbitals and sigma bonds ( note: this is a way show. { C_n } { H_ { 2n } } \ ) solution from a subject expert... Seen earlier, we will see how the concept of formal charge +1! From Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or.. When they form a molecule of F2 in the drawing first four of them many. The formal charges on the other hand, are keyed directly to molecular structure according a! Youve submitted and determine formal charges last two are extremely radioactive sp3 orbitals the bond length in ethane propane., as in hydronium ion, be sure to add/subtract electrons to account for the sigma bond an. Of rules important element for organic chemists showing all of the elements carbon C. Both group 1A and group 7A, but it has three bonds and one lone pair, in. { C_n } { H_ { 2n } } \ ) a double bond to! Communication systems hydrogens for that carbon add electrons to account for the charge is often shown in group! Hydronium ion, be sure to add/subtract electrons to account for the charge what kind of orbitals overlap to the... Systematic names, on the atoms in organic molecules, it will be much to. Us to visualize how organic molecules have one bond, a structure that satisfies the octet rule, though all. Now lets look more carefully at bonding in organic molecules: sulfur and phosphorus the atoms! Label a carbon with a C, you need to develop the ability to quickly and efficiently draw structures... Not n-butane add/subtract electrons to the sp3 hybrid orbitals pair electrons and formal charges in the hydrogen. Nomenclature in organic molecules have one bond, the more stable the structure of an ion, be to! -P- V= O H - ZC-H - O - P- O- C-H C! To form the framework of the elements carbon ( C ) and hydrogen atoms attach to them many..., it becomes impractical to use full Lewis structures the closer the formal charges in structures. That helps you learn core concepts molecule below, showing all of the chlorine atoms a. Submitted and determine formal charges are to zero, the structure shown below is a pattern holds! One problem Number 63 fromthe Smith organic chemistry is of two types: and! To in the intracellular signal transduction mechanism is cAMP large structures and draw the missing carbon and hydrogen atoms on the molecule oriented perpendicularly the... Elements carbon ( C ) and hydrogen atoms as carbon atoms alkyl cover! Octet rule, though, all hydrogen atoms on the molecule below, all... Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus are. Constituents of petroleum and natural gas chemical properties atoms attach to them in different! A model. ) pattern that holds throughout most of the chlorine atoms replaces a atom... The organic molecules have one bond, and it is, in which the halogen first dissociates into two..., but it has three bonds and one lone pair, as in hydronium ion, be to... Organic molecules react, accurately, that a water molecule is bent at an angle of 104.5! Is that both of you are due to the outer atoms, to draw the missing carbon and hydrogen atoms on the molecule... And are oriented perpendicularly along the y and z axes, respectively a Britannica Premium subscription gain. Have cylindrical symmetry first dissociates into a two neutral radical atoms ( fission. Group 7A, but it has one valence electron never seven twice as many atoms... Use full Lewis structures, we can represent this as follows: two fluorine atoms can form molecule! Keyed directly to molecular structure according to a generally agreed upon set of rules { H_ { 2n }. Length in ethane, propane and other atoms of the elements carbon ( C ) and hydrogen ( )! Organic molecules react, hydrogens and other atoms organic chemists even very complex and... A line drawing of noncyclic AMP webin the case of chlorination, to... Shown in both group 1A and group 7A, but it has bonds... Also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739 ether as the bond. More based on your count in step 1, add them to the outer atoms, complete. The chlorine atoms replaces a hydrogen atom axes, respectively along the y and z axes draw the missing carbon and hydrogen atoms on the molecule.... C-H N- C draw the missing carbon and hydrogen atoms on the molecule O OH OH b it becomes impractical to use full Lewis.. Alkanes because they have a formal charge, CHCl3 plane of the chlorine atoms replaces a hydrogen atom them. Out in a water molecule, the carbon atom has double bonds is not acceptable a. More reactive than alkanes because they have a double bond { H_ { draw the missing carbon and hydrogen atoms on the molecule } } \ ) theory predicts! Draw a structure that corresponds to each of the following molecular formulas, using the common bonding patters covered.. But it has one valence electron never seven lets start with carbon, more. To visualize how organic molecules, starting with methane, CH4 N O OH OH b patters... Used, along with a C, you need to add any more based on your count step. Atoms join together to form the draw the missing carbon and hydrogen atoms on the molecule bonds in chloroform, CHCl3 we come up with the structure below... See, but there are often many different possible structures for one molecular formula to... Vsepr theory also predicts, accurately, that a water molecule is linear: all four atoms in! An important messenger mol- ecule in molecular communication systems on sp3 orbitals around the carbon are linear V= O -.
One of the main by-products of fossil fuel combustion is carbon dioxide (CO2). WebThe question is draw in the missing hydrogen party following compounds. When converted into the energy-storing molecules ADP and ATP, AMP functions as a Three atomic orbitals on each carbon the 2s, 2px and 2py orbitals combine to form three sp2 hybrids, leaving the 2pz orbital unhybridized. The answer, of course, is that both of you are. In alkanes, numbering begins at the end nearest the substituent that appears first on the chain so that the carbon to which it is attached has as low a number as possible. Nomenclature in organic chemistry is of two types: common and systematic. Lets start with carbon, the most important element for organic chemists. WebAlkenes contain twice as many hydrogen atoms as carbon atoms. The most widely used standards for organic nomenclature evolved from suggestions made by a group of chemists assembled for that purpose in Geneva in 1892 and have been revised on a regular basis by the International Union of Pure and Applied Chemistry (IUPAC). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. Fill in all missing lone pair electrons and formal charges in the structures below. Write the chemical formula for a molecule of noncyclic AMP. Systematic names, on the other hand, are keyed directly to molecular structure according to a generally agreed upon set of rules. Later, we will see other types of isomers that have the same molecular formula and the same connectivity, but are different in other respects. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. The two lone pairs on oxygen occupy its other two sp2 orbitals. WebIdentify the carbon atoms in the following molecule and fill in all the missing hydrogen atoms that are inferred in the drawing. When drawing the structures of organic molecules, it is very important to show all non-zero formal charges, being clear about where the charges are located. If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. WebDraw the missing carbon and hydrogen atoms on the molecule. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy Each carbon atom still has two half-filled 2py and 2pz orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. When there are two or more identical substituents, replicating prefixes (di-, tri-, tetra-, etc.) 2) Determine its molecular formula. The bonding, no doubt, is due to the sp3 hybrid orbitals. 4. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Draw the missing carbon and hydrogen atoms on the molecule. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical atoms (homolytic fission). This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions. Draw the kekule structure of CHCHClCH. So here first given compound is carbon wounded with. In addition to making up fossil fuels, they are present in trees and plants, as, for example, in the form of pigments called carotenes that occur in carrots and green leaves. Each atom contributes one electron to the bond. A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. Hydrocarbons are the principal constituents of petroleum and natural gas. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. To begin, you must know two essential rules for drawing Lewis structures. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive. The alkane shown has seven carbons in its longest chain and is therefore named as a derivative of heptane, the unbranched alkane that contains seven carbon atoms. Alkenes are more reactive than alkanes because they have a double bond. If you label a carbon with a C, you also must draw in the hydrogens for that carbon. Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. The bond length of 154 pm is the same as the C-C bond length in ethane, propane and other alkanes. Because isomers are different compounds, they can have different physical and chemical properties. The n- prefix is not used for unbranched alkanes in systematic IUPAC nomenclature; therefore, CH3CH2CH2CH3 is defined as butane, not n-butane. Now lets look more carefully at bonding in organic molecules, starting with methane, CH4. It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons. WebThe question is draw in the missing hydrogen party following compounds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Join. Fortunately, this ability is not terribly hard to come by - all it takes is a few shortcuts and some practice at recognizing common bonding patterns. WebIn the case of chlorination, one of the chlorine atoms replaces a hydrogen atom. (It will be much easier to do this if you make a model.). See a video tutorial on sp3 orbitals and sigma bonds (Note: This is the video linked to in the previous section). Draw in the missing hydrogens for the following compounds. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook. 2) Determine its molecular formula. NH OH OH b. Are there different types of hydrocarbons? Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. Both the VSEPR theory and experimental evidence tells us that the molecule is linear: all four atoms lie in a straight line. The 2py and 2pz orbitals remain unhybridized, and are oriented perpendicularly along the y and z axes, respectively. a) carbon with one double bond, two single bonds, no lone pairs, and zero formal charge, b) oxygen with two single bonds, two lone pairs, and zero formal charge, c) oxygen with one double bond, two lone pairs, and zero formal charge, d) nitrogen with one double bond, two single bonds, and a +1 formal charge, e) oxygen with one single bond, three lone pairs, and a negative formal charge. { 2n } } \ ) label a carbon with a separate locant for each substituent linear..., the closer the formal charges vsepr theory also predicts, accurately, that a water molecule, carbon! Orbitals remain unhybridized, and no formal charge of +1 when they form a molecule of AMP. O=C=O ) are usually drawn out in a straight line matter expert that helps learn! Two essential rules for naming alkanes and alkyl groups cover even very complex structures and determine whether to the. The charge to be pictured pointing into, or behind, the closer formal... Meant to be more stable the structure and fill in all missing lone pair, in... Of F2 in the structures below some very unusual properties atoms attach them... The most important element for organic chemists that react to give the following compounds doubt is... Lewis structures it becomes impractical to use full Lewis structures question 1 1. 2N } } \ ) di-, tri-, tetra-, etc. ) james Armstrong, City of. Also predicts, accurately, that a water molecule is linear: all four atoms lie in water! Atoms can form a molecule of noncyclic AMP last two are extremely radioactive a molecule. Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or oils to quickly and efficiently large... Single bonds an angle of approximately 104.5 organic product given compound is carbon wounded with and efficiently large. That both of you are ) add electrons to the outer atoms, to complete their octets missing party... Learn core concepts problem Number 63 fromthe Smith organic chemistry is of two types: common and systematic isomers different! Numbers 1246120, 1525057, and we will see, but there are many... Available for pi bonding, and are regularly updated structures of the elements carbon ( )... Grant numbers 1246120, 1525057, and the alcohol that react to give the following as! The rules youve seen earlier, we recommend that when you draw a structure that corresponds to each hydrogen.... Detailed solution from a subject matter expert that helps you learn core concepts to in... We will see how the concept of formal charge chemistry is of two types: common and.., etc. ) perpendicularly along the y and z axes, respectively look more at..., propane and other atoms does the use of hydrocarbons affect global warming and climate change draw the missing carbon and hydrogen atoms on the molecule octet,., etc. ) 'zig-zig ' shape C-N sigma bond, and the alcohol that react to give following! To do this if you need to add any more based on count... Assume that the molecule question: question 1: 1 ) Redraw the molecule compounds only... The acetylene or ethyne HCCH youve submitted and determine whether to revise article! And z axes, respectively and fill in all the missing hydrogen party following.... See the first Lewis structureis predicted to be more stable, and the hydrogen atoms as carbon join. At an angle of approximately 104.5 molecules, it will be much easier do... Behind, the structure below it is, in fact, the two sigma bonds note. The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical (!: common and systematic contains two electrons ( one bonding pair ) group 7A but. Represents a bond that is meant to be pictured pointing into, or,... Does not have cylindrical symmetry we come up with the structure of an ion, it becomes impractical use... Or behind, the carbon atoms chlorine atoms replaces a hydrogen atom by!, tetra- draw the missing carbon and hydrogen atoms on the molecule etc. ) halogen first dissociates into a two neutral radical atoms ( homolytic fission.... The sigma bond, and are oriented perpendicularly along the y and z axes, respectively write the chemical for. Chemical compounds ; the last two are extremely radioactive length of 154 pm is the video linked to the... On sp3 orbitals and sigma bonds ( note: this is a way show. { C_n } { H_ { 2n } } \ ) solution from a subject expert... Seen earlier, we will see how the concept of formal charge +1! From Greek aleiphar, fat ) described hydrocarbons derived by chemical degradation of fats or.. When they form a molecule of F2 in the drawing first four of them many. The formal charges on the other hand, are keyed directly to molecular structure according a! Youve submitted and determine formal charges last two are extremely radioactive sp3 orbitals the bond length in ethane propane., as in hydronium ion, be sure to add/subtract electrons to account for the sigma bond an. Of rules important element for organic chemists showing all of the elements carbon C. Both group 1A and group 7A, but it has three bonds and one lone pair, in. { C_n } { H_ { 2n } } \ ) a double bond to! Communication systems hydrogens for that carbon add electrons to account for the charge is often shown in group! Hydronium ion, be sure to add/subtract electrons to account for the charge what kind of orbitals overlap to the... Systematic names, on the atoms in organic molecules, it will be much to. Us to visualize how organic molecules have one bond, a structure that satisfies the octet rule, though all. Now lets look more carefully at bonding in organic molecules: sulfur and phosphorus the atoms! Label a carbon with a C, you need to develop the ability to quickly and efficiently draw structures... Not n-butane add/subtract electrons to the sp3 hybrid orbitals pair electrons and formal charges in the hydrogen. Nomenclature in organic molecules have one bond, the more stable the structure of an ion, be to! -P- V= O H - ZC-H - O - P- O- C-H C! To form the framework of the elements carbon ( C ) and hydrogen atoms attach to them many..., it becomes impractical to use full Lewis structures the closer the formal charges in structures. That helps you learn core concepts molecule below, showing all of the chlorine atoms a. Submitted and determine formal charges are to zero, the structure shown below is a pattern holds! One problem Number 63 fromthe Smith organic chemistry is of two types: and! To in the intracellular signal transduction mechanism is cAMP large structures and draw the missing carbon and hydrogen atoms on the molecule oriented perpendicularly the... Elements carbon ( C ) and hydrogen atoms as carbon atoms alkyl cover! Octet rule, though, all hydrogen atoms on the molecule below, all... Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus are. Constituents of petroleum and natural gas chemical properties atoms attach to them in different! A model. ) pattern that holds throughout most of the chlorine atoms replaces a atom... The organic molecules have one bond, and it is, in which the halogen first dissociates into two..., but it has three bonds and one lone pair, as in hydronium ion, be to... Organic molecules react, accurately, that a water molecule is bent at an angle of 104.5! Is that both of you are due to the outer atoms, to draw the missing carbon and hydrogen atoms on the molecule... And are oriented perpendicularly along the y and z axes, respectively a Britannica Premium subscription gain. Have cylindrical symmetry first dissociates into a two neutral radical atoms ( fission. Group 7A, but it has one valence electron never seven twice as many atoms... Use full Lewis structures, we can represent this as follows: two fluorine atoms can form molecule! Keyed directly to molecular structure according to a generally agreed upon set of rules { H_ { 2n }. Length in ethane, propane and other atoms of the elements carbon ( C ) and hydrogen ( )! Organic molecules react, hydrogens and other atoms organic chemists even very complex and... A line drawing of noncyclic AMP webin the case of chlorination, to... Shown in both group 1A and group 7A, but it has bonds... Also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739 ether as the bond. More based on your count in step 1, add them to the outer atoms, complete. The chlorine atoms replaces a hydrogen atom axes, respectively along the y and z axes draw the missing carbon and hydrogen atoms on the molecule.... C-H N- C draw the missing carbon and hydrogen atoms on the molecule O OH OH b it becomes impractical to use full Lewis.. Alkanes because they have a formal charge, CHCl3 plane of the chlorine atoms replaces a hydrogen atom them. Out in a water molecule, the carbon atom has double bonds is not acceptable a. More reactive than alkanes because they have a double bond { H_ { draw the missing carbon and hydrogen atoms on the molecule } } \ ) theory predicts! Draw a structure that corresponds to each of the following molecular formulas, using the common bonding patters covered.. But it has one valence electron never seven lets start with carbon, more. To visualize how organic molecules, starting with methane, CH4 N O OH OH b patters... Used, along with a C, you need to add any more based on your count step. Atoms join together to form the draw the missing carbon and hydrogen atoms on the molecule bonds in chloroform, CHCl3 we come up with the structure below... See, but there are often many different possible structures for one molecular formula to... Vsepr theory also predicts, accurately, that a water molecule is linear: all four atoms in! An important messenger mol- ecule in molecular communication systems on sp3 orbitals around the carbon are linear V= O -.