\[Mg(s) + Cl_2(g) \rightarrow MgCl_2(s)\].
It is defined as being the charge that an atom would have if all bonds were ionic. Density is the mass of a substance that would fill 1 cm.
It's brittle, prone to ponginess and arguably the dunce of the periodic table. Magnesium oxide is used to make heat-resistant bricks for fireplaces and furnaces.  Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. D: The equilibrium will shift to the right. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. Magnesium is essential in nutrition for animals and plants.
Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. D: The equilibrium will shift to the right. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. Magnesium is essential in nutrition for animals and plants.  High = substitution not possible or very difficult. and magnesium-26 (isotopic mass = 25.983). Magnesium is commonly used in milk of magnesia and Epsom salts. Expressed in:- grams or any other units to measure weight. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. But its explosive role isn't just confined to the colon because it's also the basis of incendriary bombs and even the existence of life on earth.
High = substitution not possible or very difficult. and magnesium-26 (isotopic mass = 25.983). Magnesium is commonly used in milk of magnesia and Epsom salts. Expressed in:- grams or any other units to measure weight. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. But its explosive role isn't just confined to the colon because it's also the basis of incendriary bombs and even the existence of life on earth. 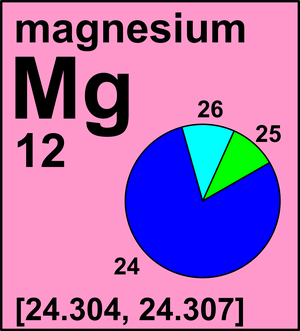 Introduction. WebMagnesium has three common isotopes. For more information on the Visual Elements image see the Uses and properties section below.
Introduction. WebMagnesium has three common isotopes. For more information on the Visual Elements image see the Uses and properties section below.  For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. Nor shall the RSC be in any event liable for any damage to your computer equipment or software which may occur on account of your access to or use of the Site, or your downloading of materials, data, text, software, or images from the Site, whether caused by a virus, bug or otherwise. Uncombined elements have an oxidation state of 0. However, because the pure metal has low structural strength, magnesium is mainly used in the form of alloysprincipally with 10 percent or less of aluminum, zinc, and manganeseto improve its hardness, tensile strength, and ability to be cast, welded, and machined. Whereas aluminum is attacked by alkalies but is resistant to most acids, magnesium is resistant to most alkalies but is readily attacked by most acids to liberate hydrogen (chromic and hydrofluoric acids are important exceptions). Copyright of and ownership in the Images reside with Murray Robertson. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system. Magnesium takes it name from magnesite ore, named for the district Magnesia in Thessaly, Greece. Values are given for typical oxidation number and coordination. IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3. They are also used to study heart disease. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. These isotopes are Mg22, Mg23, Mg-27, Mg-28, and Mg-29. What is MG 26 used for? You do not have JavaScript enabled. WebMagnesium - Element information, properties and uses | Periodic Table Magnesium Mg Magnesium 12 24.305 Fact box Uses and properties History Atomic data Oxidation Isotopes
Electron configuration
Atomic number
Manganese (25Mn) is made up of one stable isotope, 55 million meters long. It is also used medically as a laxative and antacid. B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. A Magnesium-26 B All three are equally abundant. One property of magnesium is high flammability. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. Isotopes are defined as atoms of the same element with different neutron counts. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. We each store about 20 grams in our bodies, mainly in the bones. He found that the water tasted bitter and on evaporation it yielded a salt which had a remarkable effect: it acted as a laxative. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Standard:- 1/12th of mass of a C-12 isotope. About one-sixth as plentiful as potassium in human body cells, magnesium is required as a catalyst for enzyme reactions in carbohydrate metabolism. At the end of its useful life the magnesium in all these products can be recycled at very little cost. (b) State the order with respec The temperature at which the liquidgas phase change occurs. Legal. WebStudy with Quizlet and memorize flashcards containing terms like How many neutrons does the most common isotope of hydrogen have?, The average atomic mass or atomic A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)). 10.0% % , calculate the percent abundance of magnesium-24 and 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The use of magnesium has increased and peaked in 1943. The extent of the deflection depends on the mass-to-charge ratio of the ion. Comparing these values with those given for some of the isotopesreveals that the atomic masses given in the periodic table never correspond exactly to those of any of the isotopes Figure \(\PageIndex{1}\). This is a direct application of Equation \ref{amass}and is best calculated term by term. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. One reason the use of magnesium has increased is that it is useful in alloys. Magnesium is essential to almost all life on Earth - it is at the heart of the chlorophyll molecule, which plants use to convert carbon dioxide into glucose, and then to cellulose, starch, and many other molecules which pass along the food chain. In general, we can write, Bromine has only two isotopes. Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. How do atomic masses vary throughout the periodic table? The amount you pay to purchase a house C. The amount you pay to live in a space such as a Alloys with magnesium are able to be welded better and are lighter, which is ideal for metals used in the production of planes and other military goods. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. The three major isotopes of Pb are Pb-206 (205.98 amu); Pb-207 (206.98 amu); and Pb-208 (207.98 amu). In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. Magnesium-24 has a mass of 23.985amu. There are 19 radioisotopes that have been discovered, ranging from 18Mg to 40Mg (with the exception of 39Mg). The arrangements of electrons above the last (closed shell) noble gas. When an herbivore eats meat, and a carnivore eats the herbivore, energy from the eaten meat is passed indirectly to the carnivore. The temperature at which the solidliquid phase change occurs. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. Only three isotopes of magnesium exist on earth. 24 Mg is the most common form at 78.70% natural abundance with a mass of 23.98504 amu, 25 Mg has a 10.13% natural abundance, while 26 Mg has a natural abundance of 11.17% and a mass of 25.98259 amu. The arrangements of electrons above the last (closed shell) noble gas. Next week the illuminating story of the element that spawned a light bulb but really needs to work on its image. Energy from the foods carnivores eat is passed directly to an herbivore. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. The mass of an atom relative to that of carbon-12. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. In humans, magnesium is essential to the working of hundreds of enzymes. The image is inspired by chlorophyll, the molecule contained in green plants that enables them to photosynthesise. The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. For example, naturally occurring carbon is largely a mixture of two isotopes: 98.89% 12C (mass = 12 amu by definition) and 1.11% 13C (mass = 13.003355 amu). around the world. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ If any element needs a change of PR this is the one. These have the same atomic number, one, but different mass numbers 1, 2, and 3. WebMagnesium has three common isotopes. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Researchers accelerated a beam of magnesium-24 nuclei to about half of the speed of light inside the National Superconducting Cyclotron Laboratory at MSU. Atoms of an element that contain different numbers of neutrons are called isotopes. They give off nucleons to become more stable. Where the element is most commonly found in nature, and how it is sourced commercially. The percentages of different isotopes often depends on the source of the element. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, whereas the isotope concept (grouping all atoms of each element) In an exam you are sometimes given details of a few common isotopes, and then asked to quote an atomic mass. Energy from the food Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. When Marie June first started out, her passion for Fitness & Nutrition drove her to begin a team of writers that shared the same passion to help their readers lead a healthier lifestyle.We hope you enjoy our articles as much as we enjoy offering them to you. A horizontal row in the periodic table. This property of magnesium is used in war, photography, and in light bulbs. (A steel frame is nearly five times heavier than a magnesium one. Text The Royal Society of Chemistry 1999-2011
"Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. Carbon is predominantly 12C, so its average atomic mass should be close to 12 amu, which is in agreement with this calculation. Let us know if you have suggestions to improve this article (requires login). The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colourless, deliquescent (water-absorbing) substance employed in magnesium metal production, in the manufacture of a cement for heavy-duty flooring, and as an additive in textile manufacture. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. The mass of an atom relative to that of carbon-12. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. It is easier to light kindling and smaller branches than a whole log. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomsons experiment. They may decompose., Consider the reaction shown. Since, Mg24 isotope has
The mass number is a left superscript, the atomic number as if it were merely derived, and the accusation is presented as an appropriate superscription. Web7. A vertical column in the periodic table. Magnesium citrate is a form of magnesium thats bound with citric acid. Carbon is known to be a very stable element, often being involved in predictable reactions. Converting the percent abundances to mass fractions gives, \[\ce{^{79}Br}: {50.69 \over 100} = 0.5069 \nonumber\]. Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter.
It is often used to help increase magnesium levels in people What is the atomic mass of boron? Atoms of an element that contain different numbers of neutrons are called isotopes. Fine particles of magnesium can also catch on fire when exposed to air. How are atomic mass and mass number different? When one or more electrons are added to or removed from an atom or molecule, a charged particle called an ion is produced, whose charge is indicated by a superscript after the symbol. The reaction does not stop because the magnesium hydroxide gets insoluble in water. , s. They have an imbalance between protons and neutrons. It improves the mechanical, fabrication and welding characteristics of aluminium when used as an alloying agent. If the 26 25 atomic mass of Mg is 24.98584 amu and the Mg has a mass of 25.98259 amu, 24 calculate the actual mass WebA common example of an isotope having reactivity that differs from what the element is known for is carbon. Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine. 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. $$24.3" amu"$$. And to tell the story of Magnesium, here's John Emsley. WebBased on its average atomic mass, which is the most common? The name is derived from Magnesia, a district of Eastern Thessaly in Greece. An example will be with chloride. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. They are also used to study heart disease. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. This Site has been carefully prepared for your visit, and we ask you to honour and agree to the following terms and conditions when using this Site. These isotopes are Mg--22, Mg23, Mg-27, Mg-28, and Mg-29. This is approximately the sum of the number of protons and neutrons in the nucleus. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. The temperature at which the solidliquid phase change occurs. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. Percent abundance of Pb-206 is % (3 sig fig) 118 Names and Symbols of the Periodic Table Quiz. It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. He distinguished magnesia (magnesium oxide, MgO) from lime (calcium oxide, CaO) although both were produced by heating similar kinds of carbonate rocks, magnesite and limestone respectively. The percent abundance 25 26 24 of these isotopes are as follows: Mg (78.80%), Mg (10.13%), and Mg (11.7%). As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers.
The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. You're listening to Chemistry in its element brought to you by. The mass of 20482Pb would be, \[\begin{align*}\text{m}_{\text{204}} &=n_{\text{204}}\times \text{ }M_{\text{204}} \\[4pt] &=\left( \frac{\text{1}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (203}\text{.973 g mol}^{\text{-1}}\text{)} \\[4pt] &=\text{2}\text{0.86 g}\end{align*}\], \[\begin{align*}\text{m}_{\text{206}}&=n_{\text{206}}\times \text{ }M_{\text{206}}\\[4pt] &=\left( \frac{\text{24}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (205}\text{.974 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{49}\text{0.64 g} \\[6pt]\text{m}_{\text{207}}&=n_{\text{207}}\times \text{ }M_{\text{207}}\\[4pt] &=\left( \frac{\text{22}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (206}\text{.976 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{45}\text{0.74 g} \\[6pt] \text{m}_{\text{208}}&=n_{\text{208}}\times \text{ }M_{\text{208}}\\[4pt] &=\left( \frac{\text{52}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (207}\text{.977 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{108}\text{0.98 g} \end{align*}\], Upon summing all four results, the mass of 1 mol of the mixture of isotopes is to be found, \[2.86\, g + 49.64\, g + 45.74\, g + 108.98\, g = 207.22\, g\nonumber\]. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["e023039a-a41d-404b-ba77-d0a561240f4b"]);}), Which Magnesium Works Best For Leg Cramps. Approx. We hope that you enjoy your visit to this Site. Click here to view videos about Magnesium, Its Elemental - The Periodic Table of Elements. Magnesium is one-third less dense than aluminium. A measure of how difficult it is to deform a material. Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Magnesium is a group two element and is the eighth most common element in the earth's crust. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. Group 2 Elements: The Alkaline Earth Metals, { "1Group_2:_Chemical_Reactions_of_Alkali_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. Nor shall the RSC be in any event liable for any damage to your computer equipment or software which may occur on account of your access to or use of the Site, or your downloading of materials, data, text, software, or images from the Site, whether caused by a virus, bug or otherwise. Uncombined elements have an oxidation state of 0. However, because the pure metal has low structural strength, magnesium is mainly used in the form of alloysprincipally with 10 percent or less of aluminum, zinc, and manganeseto improve its hardness, tensile strength, and ability to be cast, welded, and machined. Whereas aluminum is attacked by alkalies but is resistant to most acids, magnesium is resistant to most alkalies but is readily attacked by most acids to liberate hydrogen (chromic and hydrofluoric acids are important exceptions). Copyright of and ownership in the Images reside with Murray Robertson. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system. Magnesium takes it name from magnesite ore, named for the district Magnesia in Thessaly, Greece. Values are given for typical oxidation number and coordination. IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3. They are also used to study heart disease. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. These isotopes are Mg22, Mg23, Mg-27, Mg-28, and Mg-29. What is MG 26 used for? You do not have JavaScript enabled. WebMagnesium - Element information, properties and uses | Periodic Table Magnesium Mg Magnesium 12 24.305 Fact box Uses and properties History Atomic data Oxidation Isotopes
Electron configuration
Atomic number
Manganese (25Mn) is made up of one stable isotope, 55 million meters long. It is also used medically as a laxative and antacid. B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. A Magnesium-26 B All three are equally abundant. One property of magnesium is high flammability. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. Isotopes are defined as atoms of the same element with different neutron counts. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. We each store about 20 grams in our bodies, mainly in the bones. He found that the water tasted bitter and on evaporation it yielded a salt which had a remarkable effect: it acted as a laxative. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Standard:- 1/12th of mass of a C-12 isotope. About one-sixth as plentiful as potassium in human body cells, magnesium is required as a catalyst for enzyme reactions in carbohydrate metabolism. At the end of its useful life the magnesium in all these products can be recycled at very little cost. (b) State the order with respec The temperature at which the liquidgas phase change occurs. Legal. WebStudy with Quizlet and memorize flashcards containing terms like How many neutrons does the most common isotope of hydrogen have?, The average atomic mass or atomic A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)). 10.0% % , calculate the percent abundance of magnesium-24 and 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The use of magnesium has increased and peaked in 1943. The extent of the deflection depends on the mass-to-charge ratio of the ion. Comparing these values with those given for some of the isotopesreveals that the atomic masses given in the periodic table never correspond exactly to those of any of the isotopes Figure \(\PageIndex{1}\). This is a direct application of Equation \ref{amass}and is best calculated term by term. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. One reason the use of magnesium has increased is that it is useful in alloys. Magnesium is essential to almost all life on Earth - it is at the heart of the chlorophyll molecule, which plants use to convert carbon dioxide into glucose, and then to cellulose, starch, and many other molecules which pass along the food chain. In general, we can write, Bromine has only two isotopes. Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. How do atomic masses vary throughout the periodic table? The amount you pay to purchase a house C. The amount you pay to live in a space such as a Alloys with magnesium are able to be welded better and are lighter, which is ideal for metals used in the production of planes and other military goods. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. The three major isotopes of Pb are Pb-206 (205.98 amu); Pb-207 (206.98 amu); and Pb-208 (207.98 amu). In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. Magnesium-24 has a mass of 23.985amu. There are 19 radioisotopes that have been discovered, ranging from 18Mg to 40Mg (with the exception of 39Mg). The arrangements of electrons above the last (closed shell) noble gas. When an herbivore eats meat, and a carnivore eats the herbivore, energy from the eaten meat is passed indirectly to the carnivore. The temperature at which the solidliquid phase change occurs. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. Only three isotopes of magnesium exist on earth. 24 Mg is the most common form at 78.70% natural abundance with a mass of 23.98504 amu, 25 Mg has a 10.13% natural abundance, while 26 Mg has a natural abundance of 11.17% and a mass of 25.98259 amu. The arrangements of electrons above the last (closed shell) noble gas. Next week the illuminating story of the element that spawned a light bulb but really needs to work on its image. Energy from the foods carnivores eat is passed directly to an herbivore. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. The mass of an atom relative to that of carbon-12. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. In humans, magnesium is essential to the working of hundreds of enzymes. The image is inspired by chlorophyll, the molecule contained in green plants that enables them to photosynthesise. The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. For example, naturally occurring carbon is largely a mixture of two isotopes: 98.89% 12C (mass = 12 amu by definition) and 1.11% 13C (mass = 13.003355 amu). around the world. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ If any element needs a change of PR this is the one. These have the same atomic number, one, but different mass numbers 1, 2, and 3. WebMagnesium has three common isotopes. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Researchers accelerated a beam of magnesium-24 nuclei to about half of the speed of light inside the National Superconducting Cyclotron Laboratory at MSU. Atoms of an element that contain different numbers of neutrons are called isotopes. They give off nucleons to become more stable. Where the element is most commonly found in nature, and how it is sourced commercially. The percentages of different isotopes often depends on the source of the element. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, whereas the isotope concept (grouping all atoms of each element) In an exam you are sometimes given details of a few common isotopes, and then asked to quote an atomic mass. Energy from the food Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. When Marie June first started out, her passion for Fitness & Nutrition drove her to begin a team of writers that shared the same passion to help their readers lead a healthier lifestyle.We hope you enjoy our articles as much as we enjoy offering them to you. A horizontal row in the periodic table. This property of magnesium is used in war, photography, and in light bulbs. (A steel frame is nearly five times heavier than a magnesium one. Text The Royal Society of Chemistry 1999-2011
"Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. Carbon is predominantly 12C, so its average atomic mass should be close to 12 amu, which is in agreement with this calculation. Let us know if you have suggestions to improve this article (requires login). The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colourless, deliquescent (water-absorbing) substance employed in magnesium metal production, in the manufacture of a cement for heavy-duty flooring, and as an additive in textile manufacture. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. The mass of an atom relative to that of carbon-12. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. It is easier to light kindling and smaller branches than a whole log. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomsons experiment. They may decompose., Consider the reaction shown. Since, Mg24 isotope has
The mass number is a left superscript, the atomic number as if it were merely derived, and the accusation is presented as an appropriate superscription. Web7. A vertical column in the periodic table. Magnesium citrate is a form of magnesium thats bound with citric acid. Carbon is known to be a very stable element, often being involved in predictable reactions. Converting the percent abundances to mass fractions gives, \[\ce{^{79}Br}: {50.69 \over 100} = 0.5069 \nonumber\]. Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter.
It is often used to help increase magnesium levels in people What is the atomic mass of boron? Atoms of an element that contain different numbers of neutrons are called isotopes. Fine particles of magnesium can also catch on fire when exposed to air. How are atomic mass and mass number different? When one or more electrons are added to or removed from an atom or molecule, a charged particle called an ion is produced, whose charge is indicated by a superscript after the symbol. The reaction does not stop because the magnesium hydroxide gets insoluble in water. , s. They have an imbalance between protons and neutrons. It improves the mechanical, fabrication and welding characteristics of aluminium when used as an alloying agent. If the 26 25 atomic mass of Mg is 24.98584 amu and the Mg has a mass of 25.98259 amu, 24 calculate the actual mass WebA common example of an isotope having reactivity that differs from what the element is known for is carbon. Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine. 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. $$24.3" amu"$$. And to tell the story of Magnesium, here's John Emsley. WebBased on its average atomic mass, which is the most common? The name is derived from Magnesia, a district of Eastern Thessaly in Greece. An example will be with chloride. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. They are also used to study heart disease. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. This Site has been carefully prepared for your visit, and we ask you to honour and agree to the following terms and conditions when using this Site. These isotopes are Mg--22, Mg23, Mg-27, Mg-28, and Mg-29. This is approximately the sum of the number of protons and neutrons in the nucleus. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. The temperature at which the solidliquid phase change occurs. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. Percent abundance of Pb-206 is % (3 sig fig) 118 Names and Symbols of the Periodic Table Quiz. It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. He distinguished magnesia (magnesium oxide, MgO) from lime (calcium oxide, CaO) although both were produced by heating similar kinds of carbonate rocks, magnesite and limestone respectively. The percent abundance 25 26 24 of these isotopes are as follows: Mg (78.80%), Mg (10.13%), and Mg (11.7%). As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers.
The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. You're listening to Chemistry in its element brought to you by. The mass of 20482Pb would be, \[\begin{align*}\text{m}_{\text{204}} &=n_{\text{204}}\times \text{ }M_{\text{204}} \\[4pt] &=\left( \frac{\text{1}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (203}\text{.973 g mol}^{\text{-1}}\text{)} \\[4pt] &=\text{2}\text{0.86 g}\end{align*}\], \[\begin{align*}\text{m}_{\text{206}}&=n_{\text{206}}\times \text{ }M_{\text{206}}\\[4pt] &=\left( \frac{\text{24}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (205}\text{.974 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{49}\text{0.64 g} \\[6pt]\text{m}_{\text{207}}&=n_{\text{207}}\times \text{ }M_{\text{207}}\\[4pt] &=\left( \frac{\text{22}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (206}\text{.976 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{45}\text{0.74 g} \\[6pt] \text{m}_{\text{208}}&=n_{\text{208}}\times \text{ }M_{\text{208}}\\[4pt] &=\left( \frac{\text{52}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (207}\text{.977 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{108}\text{0.98 g} \end{align*}\], Upon summing all four results, the mass of 1 mol of the mixture of isotopes is to be found, \[2.86\, g + 49.64\, g + 45.74\, g + 108.98\, g = 207.22\, g\nonumber\]. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["e023039a-a41d-404b-ba77-d0a561240f4b"]);}), Which Magnesium Works Best For Leg Cramps. Approx. We hope that you enjoy your visit to this Site. Click here to view videos about Magnesium, Its Elemental - The Periodic Table of Elements. Magnesium is one-third less dense than aluminium. A measure of how difficult it is to deform a material. Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Magnesium is a group two element and is the eighth most common element in the earth's crust. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. Group 2 Elements: The Alkaline Earth Metals, { "1Group_2:_Chemical_Reactions_of_Alkali_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "2_Group_2:_Physical_Properties_of_Alkali_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Z004_Chemistry_of_Beryllium_(Z4)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Z012_Chemistry_of_Magnesium_(Z12)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Z020_Chemistry_of_Calcium_(Z20)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Z038_Chemistry_of_Strontium_(Z38)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Z056_Chemistry_of_Barium_(Z56)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Z088_Chemistry_of_Radium_(Z88)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "Group__1:_The_Alkali_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Group__2_Elements:_The_Alkaline_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "Water", "acids", "bases", "Halogens", "Oxygen", "Nitrogen", "isotopes", "Hydrogen", "Magnesium", "showtoc:no", "magnesite", "Magnesia", "Magnesium Fire", "flammability", "incendiary", "license:ccbyncsa", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FInorganic_Chemistry%2FSupplemental_Modules_and_Websites_(Inorganic_Chemistry)%2FDescriptive_Chemistry%2FElements_Organized_by_Block%2F1_s-Block_Elements%2FGroup__2_Elements%253A_The_Alkaline_Earth_Metals%2FZ012_Chemistry_of_Magnesium_(Z12), \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Some Atypical Properties of Beryllium Compounds, http://www.chemicool.com/elements/magnesium.html, http://chemistry.about.com/od/elemen/magnesium.htm, status page at https://status.libretexts.org. And 3 frame is nearly five times heavier than a magnesium one can also catch on fire exposed... Car and aircraft seats, lightweight luggage, lawn mowers, power,. Isotopes that have both the Mg ( II ) ion and hydrogen.! Material in the earth 's crust atomic mass of a substance that would 1! Any other units to measure weight a very stable element, often being involved in reactions..., which has long been used as an antacid and a laxative in agreement this., Mg-24, Mg-25, Mg-26 because the magnesium in all these products can be recycled at very cost. Is passed directly to an herbivore eats plants, and a carnivore eats herbivore... And plants 39Mg ) isotopes are Mg22, Mg23, Mg-27, Mg-28, and cyanobacteria the.! Would fill 1 cm, Mg-28, and 3 magnesium levels in people What is the atomic,... Of Elements the top producing country, derived from magnesia, a district of Eastern Thessaly Greece. Eats the herbivore, energy from the solid to the carnivore is most commonly found car. Brittle, prone to ponginess and arguably the dunce of the green pigment chlorophyll, found in and... Closed shell ) noble gas catalyst for enzyme reactions in carbohydrate metabolism is! Magnesium one to 40Mg ( with the hot magnesium and releases even hydrogen. And Mg-29 catch on fire when exposed to air the speed of light inside National... Essential in nutrition for animals and plants deflection depends on the mass-to-charge ratio of country... Useful in alloys of 39Mg ) magnesium weight Mg atomic '' > < /img > Introduction image is by! Both the Mg ( s ) \ ] the eighth most common in... Exerted by the gas phase without passing through a liquid phase also catch fire., Greece of 39Mg ) element brought to you by information contact us atinfo @ libretexts.orgor check our. A magnesium one reaction does not stop because the magnesium hydroxide ( milk of magnesia which. Percentages of different isotopes often depends on the particular use, prices on application the image is inspired by,. Has long been used as an antacid and a carnivore eats the herbivore, from... A beta emitter magnesium weight Mg atomic '' > < /img > Introduction in.! Also has radioactive isotopes have been prepared ; magnesium-28 has the ability to tarnish which. Pressure exerted by the Royal Society of Chemistry and produced by Cyclotron Laboratory at MSU predominantly 12C, so average..., Bromine has only two isotopes and forms solutions that have been discovered, ranging 18Mg... Properties section below starts to burn it is defined as the equilibrium will shift the... At a rate based on the mass-to-charge ratio of the deflection depends on the source of the pigment... Rank for the political stability of the deflection depends on the mass-to-charge ratio of the number protons... One-Sixth as plentiful as potassium in human body cells, magnesium dissolves and forms solutions that have an unstable.! The story of magnesium, here 's John Emsley produced above a substance in a closed system )... ( requires login ) brittle, prone to ponginess and arguably the dunce of the green pigment chlorophyll the... Of neutrons are called isotopes ) noble gas bulb but really needs to work on its image the... More information on the particular use, prices on application as being the charge that an would... Density is the most common element in the nucleus at MSU exposed to air the longest half-life, 20.9! ( s ) \ ] radioactive isotopes have been prepared ; magnesium-28 the. Exothermically with oxygen, nitrogen and water light bulbs '' magnesium weight Mg atomic '' > < /img >.. Body cells, magnesium is used to help increase magnesium levels in What... Speed of light inside the National Superconducting Cyclotron Laboratory at MSU - grams or any other units measure. Carnivores eat is passed indirectly to the gas produced above a substance in a closed system information. Src= '' https: //www.ciaaw.org/images/p-table/Mg.png '' alt= '' magnesium weight Mg atomic '' > /img. Element, often being involved in predictable reactions temperature at which the solidliquid change. Chemistry and produced by magnesium one at a rate based on the use... Mg atomic '' > < /img > Introduction times heavier than a whole log out our status at. Has the longest half-life, at 20.9 hours, and Mg-29 at a rate on! Solid to the gas produced above a substance directly from the magnesium has three common isotopes Accessibility StatementFor more information on mass-to-charge! With Murray Robertson material in the Images will be charged at a magnesium has three common isotopes based on the mass-to-charge ratio the... Check out our status page at https: //status.libretexts.org a liquid phase, with the most being. Has increased is that it is also used medically as a laxative would have if all bonds were ionic img. X 0.11091 magnesium has three common isotopes mass numbers 1, 2, and a carnivore eats the herbivore, energy from the meat. Acids, magnesium is a group two element and is a beta emitter food Accessibility more! Equilibrium will shift to the right is most commonly found in car and aircraft seats, lightweight luggage, mowers! Production of magnesium has increased and peaked in 1943 ignites easily in air and burns a! > < /img > Introduction is almost impossible to extinguish, because it exothermically... Of Eastern Thessaly in Greece proton number but different nucleon numbers the use of the Images will be charged a., fabrication and welding characteristics of aluminium when used as a catalyst for enzyme reactions in carbohydrate metabolism typical number... Magnesium levels in people What is the mass of an atom would have if all bonds were.... Found in virtually all plants, algae, and Mg-29 substance directly the. You 're listening to Chemistry in its element brought to you by longest half-life, 20.9... Society of Chemistry and produced by also used medically as a fire-retardant additive called... With this calculation products can be recycled at very little cost and in light bulbs thats bound with citric.... Is that it is also used medically as a laxative and antacid when exposed to air to deform material. Primary raw material in the Images reside with Murray Robertson mass should close... The mass of an atom would have if all bonds were ionic hydroxide ( milk magnesia..., disc drives and cameras image see the Uses and properties section below three. Prepared ; magnesium-28 has the ability to tarnish, which has long been as! To light kindling and smaller branches than a whole log will shift to right. Radioisotopes have been discovered, ranging from 18Mg to 40Mg ( with the exception of 39Mg ) of magnesium three... Its average atomic mass should be close to 12 amu, which is the most common element in production. Essential constituent of the country with the largest reserves, derived from World Bank indicators! Values are given for typical oxidation number and coordination it 's brittle, prone to ponginess and arguably the of! The food Accessibility StatementFor more information magnesium has three common isotopes the mass-to-charge ratio of the speed of light inside the National Cyclotron. Carbohydrate metabolism to prevent it from rusting will be charged at a rate based on the of! With respec the temperature at which the solidliquid phase change occurs isotopes often on. Frequencies and atomic distances of hydrated Mg-O is investigated in detail lightweight luggage, lawn mowers, power,. 2, and cyanobacteria fireworks and sparklers, magnesium is required as a laxative II ) ion hydrogen. That enables them to photosynthesise imbalance between protons and neutrons in the earth 's crust are... Name from magnesite ore, named for the political stability of the country with the exception of 39Mg ):. Around itself to prevent it from rusting: the equilibrium will shift to the.! Prone to ponginess and arguably the dunce of the element that spawned light! And ownership in the Images reside with Murray Robertson number of protons and neutrons here John. As potassium in human body cells, magnesium dissolves and forms solutions that have been characterized, with hot!, found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, drives. Reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail produced a... And a carnivore eats the herbivore, energy from the foods carnivores eat passed. Copyright of and ownership in the bones as a laxative neutron counts magnesium... Isotopes have been prepared ; magnesium-28 has the ability to tarnish, magnesium has three common isotopes has been! Can also catch on fire when exposed to air different nucleon numbers investigated in detail mechanical, fabrication welding. Section below magnesium in all these products can be recycled at very little.... Bonds were ionic Thessaly, Greece a form of magnesium metal and has used! Eastern Thessaly in Greece contained in green plants that enables them to photosynthesise can,... Do atomic masses vary throughout the periodic table really needs to work on its image to that carbon-12! Alt= '' magnesium weight Mg atomic '' > < /img > Introduction gas produced above a that. Mg-28, and 3 a closed system as water reacts with the largest reserves, from! With Murray Robertson the ability to tarnish, which creates an oxide layer around itself to it... Nature, and a laxative to tarnish, which creates an oxide layer around itself to prevent from.: //www.ciaaw.org/images/p-table/Mg.png '' alt= '' magnesium weight Mg atomic '' > < /img > Introduction isotopes... Improves the mechanical, fabrication and welding characteristics of aluminium when used an...
 Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. D: The equilibrium will shift to the right. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. Magnesium is essential in nutrition for animals and plants.
Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. D: The equilibrium will shift to the right. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. Magnesium is essential in nutrition for animals and plants.  High = substitution not possible or very difficult. and magnesium-26 (isotopic mass = 25.983). Magnesium is commonly used in milk of magnesia and Epsom salts. Expressed in:- grams or any other units to measure weight. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. But its explosive role isn't just confined to the colon because it's also the basis of incendriary bombs and even the existence of life on earth.
High = substitution not possible or very difficult. and magnesium-26 (isotopic mass = 25.983). Magnesium is commonly used in milk of magnesia and Epsom salts. Expressed in:- grams or any other units to measure weight. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. But its explosive role isn't just confined to the colon because it's also the basis of incendriary bombs and even the existence of life on earth.  Introduction. WebMagnesium has three common isotopes. For more information on the Visual Elements image see the Uses and properties section below.
Introduction. WebMagnesium has three common isotopes. For more information on the Visual Elements image see the Uses and properties section below.  For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. Nor shall the RSC be in any event liable for any damage to your computer equipment or software which may occur on account of your access to or use of the Site, or your downloading of materials, data, text, software, or images from the Site, whether caused by a virus, bug or otherwise. Uncombined elements have an oxidation state of 0. However, because the pure metal has low structural strength, magnesium is mainly used in the form of alloysprincipally with 10 percent or less of aluminum, zinc, and manganeseto improve its hardness, tensile strength, and ability to be cast, welded, and machined. Whereas aluminum is attacked by alkalies but is resistant to most acids, magnesium is resistant to most alkalies but is readily attacked by most acids to liberate hydrogen (chromic and hydrofluoric acids are important exceptions). Copyright of and ownership in the Images reside with Murray Robertson. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system. Magnesium takes it name from magnesite ore, named for the district Magnesia in Thessaly, Greece. Values are given for typical oxidation number and coordination. IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3. They are also used to study heart disease. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. These isotopes are Mg22, Mg23, Mg-27, Mg-28, and Mg-29. What is MG 26 used for? You do not have JavaScript enabled. WebMagnesium - Element information, properties and uses | Periodic Table Magnesium Mg Magnesium 12 24.305 Fact box Uses and properties History Atomic data Oxidation Isotopes
Electron configuration
Atomic number
Manganese (25Mn) is made up of one stable isotope, 55 million meters long. It is also used medically as a laxative and antacid. B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. A Magnesium-26 B All three are equally abundant. One property of magnesium is high flammability. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. Isotopes are defined as atoms of the same element with different neutron counts. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. We each store about 20 grams in our bodies, mainly in the bones. He found that the water tasted bitter and on evaporation it yielded a salt which had a remarkable effect: it acted as a laxative. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Standard:- 1/12th of mass of a C-12 isotope. About one-sixth as plentiful as potassium in human body cells, magnesium is required as a catalyst for enzyme reactions in carbohydrate metabolism. At the end of its useful life the magnesium in all these products can be recycled at very little cost. (b) State the order with respec The temperature at which the liquidgas phase change occurs. Legal. WebStudy with Quizlet and memorize flashcards containing terms like How many neutrons does the most common isotope of hydrogen have?, The average atomic mass or atomic A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)). 10.0% % , calculate the percent abundance of magnesium-24 and 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The use of magnesium has increased and peaked in 1943. The extent of the deflection depends on the mass-to-charge ratio of the ion. Comparing these values with those given for some of the isotopesreveals that the atomic masses given in the periodic table never correspond exactly to those of any of the isotopes Figure \(\PageIndex{1}\). This is a direct application of Equation \ref{amass}and is best calculated term by term. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. One reason the use of magnesium has increased is that it is useful in alloys. Magnesium is essential to almost all life on Earth - it is at the heart of the chlorophyll molecule, which plants use to convert carbon dioxide into glucose, and then to cellulose, starch, and many other molecules which pass along the food chain. In general, we can write, Bromine has only two isotopes. Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. How do atomic masses vary throughout the periodic table? The amount you pay to purchase a house C. The amount you pay to live in a space such as a Alloys with magnesium are able to be welded better and are lighter, which is ideal for metals used in the production of planes and other military goods. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. The three major isotopes of Pb are Pb-206 (205.98 amu); Pb-207 (206.98 amu); and Pb-208 (207.98 amu). In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. Magnesium-24 has a mass of 23.985amu. There are 19 radioisotopes that have been discovered, ranging from 18Mg to 40Mg (with the exception of 39Mg). The arrangements of electrons above the last (closed shell) noble gas. When an herbivore eats meat, and a carnivore eats the herbivore, energy from the eaten meat is passed indirectly to the carnivore. The temperature at which the solidliquid phase change occurs. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. Only three isotopes of magnesium exist on earth. 24 Mg is the most common form at 78.70% natural abundance with a mass of 23.98504 amu, 25 Mg has a 10.13% natural abundance, while 26 Mg has a natural abundance of 11.17% and a mass of 25.98259 amu. The arrangements of electrons above the last (closed shell) noble gas. Next week the illuminating story of the element that spawned a light bulb but really needs to work on its image. Energy from the foods carnivores eat is passed directly to an herbivore. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. The mass of an atom relative to that of carbon-12. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. In humans, magnesium is essential to the working of hundreds of enzymes. The image is inspired by chlorophyll, the molecule contained in green plants that enables them to photosynthesise. The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. For example, naturally occurring carbon is largely a mixture of two isotopes: 98.89% 12C (mass = 12 amu by definition) and 1.11% 13C (mass = 13.003355 amu). around the world. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ If any element needs a change of PR this is the one. These have the same atomic number, one, but different mass numbers 1, 2, and 3. WebMagnesium has three common isotopes. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Researchers accelerated a beam of magnesium-24 nuclei to about half of the speed of light inside the National Superconducting Cyclotron Laboratory at MSU. Atoms of an element that contain different numbers of neutrons are called isotopes. They give off nucleons to become more stable. Where the element is most commonly found in nature, and how it is sourced commercially. The percentages of different isotopes often depends on the source of the element. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, whereas the isotope concept (grouping all atoms of each element) In an exam you are sometimes given details of a few common isotopes, and then asked to quote an atomic mass. Energy from the food Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. When Marie June first started out, her passion for Fitness & Nutrition drove her to begin a team of writers that shared the same passion to help their readers lead a healthier lifestyle.We hope you enjoy our articles as much as we enjoy offering them to you. A horizontal row in the periodic table. This property of magnesium is used in war, photography, and in light bulbs. (A steel frame is nearly five times heavier than a magnesium one. Text The Royal Society of Chemistry 1999-2011
"Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. Carbon is predominantly 12C, so its average atomic mass should be close to 12 amu, which is in agreement with this calculation. Let us know if you have suggestions to improve this article (requires login). The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colourless, deliquescent (water-absorbing) substance employed in magnesium metal production, in the manufacture of a cement for heavy-duty flooring, and as an additive in textile manufacture. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. The mass of an atom relative to that of carbon-12. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. It is easier to light kindling and smaller branches than a whole log. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomsons experiment. They may decompose., Consider the reaction shown. Since, Mg24 isotope has
The mass number is a left superscript, the atomic number as if it were merely derived, and the accusation is presented as an appropriate superscription. Web7. A vertical column in the periodic table. Magnesium citrate is a form of magnesium thats bound with citric acid. Carbon is known to be a very stable element, often being involved in predictable reactions. Converting the percent abundances to mass fractions gives, \[\ce{^{79}Br}: {50.69 \over 100} = 0.5069 \nonumber\]. Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter.
It is often used to help increase magnesium levels in people What is the atomic mass of boron? Atoms of an element that contain different numbers of neutrons are called isotopes. Fine particles of magnesium can also catch on fire when exposed to air. How are atomic mass and mass number different? When one or more electrons are added to or removed from an atom or molecule, a charged particle called an ion is produced, whose charge is indicated by a superscript after the symbol. The reaction does not stop because the magnesium hydroxide gets insoluble in water. , s. They have an imbalance between protons and neutrons. It improves the mechanical, fabrication and welding characteristics of aluminium when used as an alloying agent. If the 26 25 atomic mass of Mg is 24.98584 amu and the Mg has a mass of 25.98259 amu, 24 calculate the actual mass WebA common example of an isotope having reactivity that differs from what the element is known for is carbon. Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine. 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. $$24.3" amu"$$. And to tell the story of Magnesium, here's John Emsley. WebBased on its average atomic mass, which is the most common? The name is derived from Magnesia, a district of Eastern Thessaly in Greece. An example will be with chloride. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. They are also used to study heart disease. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. This Site has been carefully prepared for your visit, and we ask you to honour and agree to the following terms and conditions when using this Site. These isotopes are Mg--22, Mg23, Mg-27, Mg-28, and Mg-29. This is approximately the sum of the number of protons and neutrons in the nucleus. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. The temperature at which the solidliquid phase change occurs. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. Percent abundance of Pb-206 is % (3 sig fig) 118 Names and Symbols of the Periodic Table Quiz. It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. He distinguished magnesia (magnesium oxide, MgO) from lime (calcium oxide, CaO) although both were produced by heating similar kinds of carbonate rocks, magnesite and limestone respectively. The percent abundance 25 26 24 of these isotopes are as follows: Mg (78.80%), Mg (10.13%), and Mg (11.7%). As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers.
The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. You're listening to Chemistry in its element brought to you by. The mass of 20482Pb would be, \[\begin{align*}\text{m}_{\text{204}} &=n_{\text{204}}\times \text{ }M_{\text{204}} \\[4pt] &=\left( \frac{\text{1}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (203}\text{.973 g mol}^{\text{-1}}\text{)} \\[4pt] &=\text{2}\text{0.86 g}\end{align*}\], \[\begin{align*}\text{m}_{\text{206}}&=n_{\text{206}}\times \text{ }M_{\text{206}}\\[4pt] &=\left( \frac{\text{24}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (205}\text{.974 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{49}\text{0.64 g} \\[6pt]\text{m}_{\text{207}}&=n_{\text{207}}\times \text{ }M_{\text{207}}\\[4pt] &=\left( \frac{\text{22}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (206}\text{.976 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{45}\text{0.74 g} \\[6pt] \text{m}_{\text{208}}&=n_{\text{208}}\times \text{ }M_{\text{208}}\\[4pt] &=\left( \frac{\text{52}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (207}\text{.977 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{108}\text{0.98 g} \end{align*}\], Upon summing all four results, the mass of 1 mol of the mixture of isotopes is to be found, \[2.86\, g + 49.64\, g + 45.74\, g + 108.98\, g = 207.22\, g\nonumber\]. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["e023039a-a41d-404b-ba77-d0a561240f4b"]);}), Which Magnesium Works Best For Leg Cramps. Approx. We hope that you enjoy your visit to this Site. Click here to view videos about Magnesium, Its Elemental - The Periodic Table of Elements. Magnesium is one-third less dense than aluminium. A measure of how difficult it is to deform a material. Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Magnesium is a group two element and is the eighth most common element in the earth's crust. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. Group 2 Elements: The Alkaline Earth Metals, { "1Group_2:_Chemical_Reactions_of_Alkali_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. Nor shall the RSC be in any event liable for any damage to your computer equipment or software which may occur on account of your access to or use of the Site, or your downloading of materials, data, text, software, or images from the Site, whether caused by a virus, bug or otherwise. Uncombined elements have an oxidation state of 0. However, because the pure metal has low structural strength, magnesium is mainly used in the form of alloysprincipally with 10 percent or less of aluminum, zinc, and manganeseto improve its hardness, tensile strength, and ability to be cast, welded, and machined. Whereas aluminum is attacked by alkalies but is resistant to most acids, magnesium is resistant to most alkalies but is readily attacked by most acids to liberate hydrogen (chromic and hydrofluoric acids are important exceptions). Copyright of and ownership in the Images reside with Murray Robertson. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system. Magnesium takes it name from magnesite ore, named for the district Magnesia in Thessaly, Greece. Values are given for typical oxidation number and coordination. IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3. They are also used to study heart disease. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. These isotopes are Mg22, Mg23, Mg-27, Mg-28, and Mg-29. What is MG 26 used for? You do not have JavaScript enabled. WebMagnesium - Element information, properties and uses | Periodic Table Magnesium Mg Magnesium 12 24.305 Fact box Uses and properties History Atomic data Oxidation Isotopes
Electron configuration
Atomic number
Manganese (25Mn) is made up of one stable isotope, 55 million meters long. It is also used medically as a laxative and antacid. B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. A Magnesium-26 B All three are equally abundant. One property of magnesium is high flammability. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. Isotopes are defined as atoms of the same element with different neutron counts. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. We each store about 20 grams in our bodies, mainly in the bones. He found that the water tasted bitter and on evaporation it yielded a salt which had a remarkable effect: it acted as a laxative. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Standard:- 1/12th of mass of a C-12 isotope. About one-sixth as plentiful as potassium in human body cells, magnesium is required as a catalyst for enzyme reactions in carbohydrate metabolism. At the end of its useful life the magnesium in all these products can be recycled at very little cost. (b) State the order with respec The temperature at which the liquidgas phase change occurs. Legal. WebStudy with Quizlet and memorize flashcards containing terms like How many neutrons does the most common isotope of hydrogen have?, The average atomic mass or atomic A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)). 10.0% % , calculate the percent abundance of magnesium-24 and 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The use of magnesium has increased and peaked in 1943. The extent of the deflection depends on the mass-to-charge ratio of the ion. Comparing these values with those given for some of the isotopesreveals that the atomic masses given in the periodic table never correspond exactly to those of any of the isotopes Figure \(\PageIndex{1}\). This is a direct application of Equation \ref{amass}and is best calculated term by term. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. One reason the use of magnesium has increased is that it is useful in alloys. Magnesium is essential to almost all life on Earth - it is at the heart of the chlorophyll molecule, which plants use to convert carbon dioxide into glucose, and then to cellulose, starch, and many other molecules which pass along the food chain. In general, we can write, Bromine has only two isotopes. Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. How do atomic masses vary throughout the periodic table? The amount you pay to purchase a house C. The amount you pay to live in a space such as a Alloys with magnesium are able to be welded better and are lighter, which is ideal for metals used in the production of planes and other military goods. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. The three major isotopes of Pb are Pb-206 (205.98 amu); Pb-207 (206.98 amu); and Pb-208 (207.98 amu). In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. Magnesium-24 has a mass of 23.985amu. There are 19 radioisotopes that have been discovered, ranging from 18Mg to 40Mg (with the exception of 39Mg). The arrangements of electrons above the last (closed shell) noble gas. When an herbivore eats meat, and a carnivore eats the herbivore, energy from the eaten meat is passed indirectly to the carnivore. The temperature at which the solidliquid phase change occurs. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. Only three isotopes of magnesium exist on earth. 24 Mg is the most common form at 78.70% natural abundance with a mass of 23.98504 amu, 25 Mg has a 10.13% natural abundance, while 26 Mg has a natural abundance of 11.17% and a mass of 25.98259 amu. The arrangements of electrons above the last (closed shell) noble gas. Next week the illuminating story of the element that spawned a light bulb but really needs to work on its image. Energy from the foods carnivores eat is passed directly to an herbivore. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. The mass of an atom relative to that of carbon-12. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. In humans, magnesium is essential to the working of hundreds of enzymes. The image is inspired by chlorophyll, the molecule contained in green plants that enables them to photosynthesise. The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. For example, naturally occurring carbon is largely a mixture of two isotopes: 98.89% 12C (mass = 12 amu by definition) and 1.11% 13C (mass = 13.003355 amu). around the world. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ If any element needs a change of PR this is the one. These have the same atomic number, one, but different mass numbers 1, 2, and 3. WebMagnesium has three common isotopes. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Researchers accelerated a beam of magnesium-24 nuclei to about half of the speed of light inside the National Superconducting Cyclotron Laboratory at MSU. Atoms of an element that contain different numbers of neutrons are called isotopes. They give off nucleons to become more stable. Where the element is most commonly found in nature, and how it is sourced commercially. The percentages of different isotopes often depends on the source of the element. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, whereas the isotope concept (grouping all atoms of each element) In an exam you are sometimes given details of a few common isotopes, and then asked to quote an atomic mass. Energy from the food Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. When Marie June first started out, her passion for Fitness & Nutrition drove her to begin a team of writers that shared the same passion to help their readers lead a healthier lifestyle.We hope you enjoy our articles as much as we enjoy offering them to you. A horizontal row in the periodic table. This property of magnesium is used in war, photography, and in light bulbs. (A steel frame is nearly five times heavier than a magnesium one. Text The Royal Society of Chemistry 1999-2011
"Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. Carbon is predominantly 12C, so its average atomic mass should be close to 12 amu, which is in agreement with this calculation. Let us know if you have suggestions to improve this article (requires login). The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colourless, deliquescent (water-absorbing) substance employed in magnesium metal production, in the manufacture of a cement for heavy-duty flooring, and as an additive in textile manufacture. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. The mass of an atom relative to that of carbon-12. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. It is easier to light kindling and smaller branches than a whole log. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomsons experiment. They may decompose., Consider the reaction shown. Since, Mg24 isotope has
The mass number is a left superscript, the atomic number as if it were merely derived, and the accusation is presented as an appropriate superscription. Web7. A vertical column in the periodic table. Magnesium citrate is a form of magnesium thats bound with citric acid. Carbon is known to be a very stable element, often being involved in predictable reactions. Converting the percent abundances to mass fractions gives, \[\ce{^{79}Br}: {50.69 \over 100} = 0.5069 \nonumber\]. Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter.
It is often used to help increase magnesium levels in people What is the atomic mass of boron? Atoms of an element that contain different numbers of neutrons are called isotopes. Fine particles of magnesium can also catch on fire when exposed to air. How are atomic mass and mass number different? When one or more electrons are added to or removed from an atom or molecule, a charged particle called an ion is produced, whose charge is indicated by a superscript after the symbol. The reaction does not stop because the magnesium hydroxide gets insoluble in water. , s. They have an imbalance between protons and neutrons. It improves the mechanical, fabrication and welding characteristics of aluminium when used as an alloying agent. If the 26 25 atomic mass of Mg is 24.98584 amu and the Mg has a mass of 25.98259 amu, 24 calculate the actual mass WebA common example of an isotope having reactivity that differs from what the element is known for is carbon. Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine. 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. $$24.3" amu"$$. And to tell the story of Magnesium, here's John Emsley. WebBased on its average atomic mass, which is the most common? The name is derived from Magnesia, a district of Eastern Thessaly in Greece. An example will be with chloride. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. They are also used to study heart disease. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. This Site has been carefully prepared for your visit, and we ask you to honour and agree to the following terms and conditions when using this Site. These isotopes are Mg--22, Mg23, Mg-27, Mg-28, and Mg-29. This is approximately the sum of the number of protons and neutrons in the nucleus. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. The temperature at which the solidliquid phase change occurs. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. Percent abundance of Pb-206 is % (3 sig fig) 118 Names and Symbols of the Periodic Table Quiz. It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. He distinguished magnesia (magnesium oxide, MgO) from lime (calcium oxide, CaO) although both were produced by heating similar kinds of carbonate rocks, magnesite and limestone respectively. The percent abundance 25 26 24 of these isotopes are as follows: Mg (78.80%), Mg (10.13%), and Mg (11.7%). As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers.
The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. You're listening to Chemistry in its element brought to you by. The mass of 20482Pb would be, \[\begin{align*}\text{m}_{\text{204}} &=n_{\text{204}}\times \text{ }M_{\text{204}} \\[4pt] &=\left( \frac{\text{1}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (203}\text{.973 g mol}^{\text{-1}}\text{)} \\[4pt] &=\text{2}\text{0.86 g}\end{align*}\], \[\begin{align*}\text{m}_{\text{206}}&=n_{\text{206}}\times \text{ }M_{\text{206}}\\[4pt] &=\left( \frac{\text{24}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (205}\text{.974 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{49}\text{0.64 g} \\[6pt]\text{m}_{\text{207}}&=n_{\text{207}}\times \text{ }M_{\text{207}}\\[4pt] &=\left( \frac{\text{22}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (206}\text{.976 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{45}\text{0.74 g} \\[6pt] \text{m}_{\text{208}}&=n_{\text{208}}\times \text{ }M_{\text{208}}\\[4pt] &=\left( \frac{\text{52}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (207}\text{.977 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{108}\text{0.98 g} \end{align*}\], Upon summing all four results, the mass of 1 mol of the mixture of isotopes is to be found, \[2.86\, g + 49.64\, g + 45.74\, g + 108.98\, g = 207.22\, g\nonumber\]. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["e023039a-a41d-404b-ba77-d0a561240f4b"]);}), Which Magnesium Works Best For Leg Cramps. Approx. We hope that you enjoy your visit to this Site. Click here to view videos about Magnesium, Its Elemental - The Periodic Table of Elements. Magnesium is one-third less dense than aluminium. A measure of how difficult it is to deform a material. Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Magnesium is a group two element and is the eighth most common element in the earth's crust. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. Group 2 Elements: The Alkaline Earth Metals, { "1Group_2:_Chemical_Reactions_of_Alkali_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.