The video from NurdRage shows how to prepare silver chloride and by exposing it to strong light how to take a negative image. A 1.50 g sample of hydrocarbon (containing C & H only) undergoes complete combustion to produce 4.40 g of CO2 and 2.70 g of H2O. 4 0 obj
 Write the molecular and net ionic equations for the reactions: Ba2+ with Na2SO4(aq). What was the concentration of each ion in the original solutions? 9
qvD9Y. \(Fe^{2+}(aq) + 2OH^-(aq) \rightarrow Fe(OH)_2(s)\), \(2PO_4^{3-}(aq) + 3Hg^{2+}(aq) \rightarrow Hg_3(PO_4)_2(s)\), \(Ca^{2+}(aq) + CO_3^{2-}(aq) \rightarrow CaCO_3(s)\). subclass of an exchange reaction that yields an insoluble product (a precipitate) when two solutions are mixed.
Write the molecular and net ionic equations for the reactions: Ba2+ with Na2SO4(aq). What was the concentration of each ion in the original solutions? 9
qvD9Y. \(Fe^{2+}(aq) + 2OH^-(aq) \rightarrow Fe(OH)_2(s)\), \(2PO_4^{3-}(aq) + 3Hg^{2+}(aq) \rightarrow Hg_3(PO_4)_2(s)\), \(Ca^{2+}(aq) + CO_3^{2-}(aq) \rightarrow CaCO_3(s)\). subclass of an exchange reaction that yields an insoluble product (a precipitate) when two solutions are mixed. 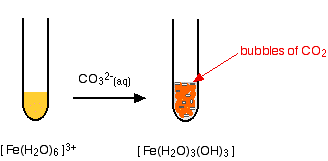 x]mo. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Provide the molecular, ionic, and net ionic equations of the following: Sulfuric acid + Sodium acetate. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Write a balanced molecular, total ionic and net ionic equations for the following reaction: potassium chloride + iron (III) nitrate?
x]mo. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Provide the molecular, ionic, and net ionic equations of the following: Sulfuric acid + Sodium acetate. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Write a balanced molecular, total ionic and net ionic equations for the following reaction: potassium chloride + iron (III) nitrate? 
 , - b p ucuuO &h{3 hU 5CJ OJ QJ \^J aJ #h{3 h{3 6CJ OJ QJ ^J aJ h{3 h{3 CJ OJ QJ ^J aJ &h{3 hU 6CJ OJ QJ ]^J aJ &h{3 h{3 5>*CJ OJ QJ ^J aJ &h{3 hU 5CJ OJ QJ \^J aJ h{3 hU CJ OJ QJ ^J aJ h{3 hU CJ OJ QJ aJ h{3 h( CJ OJ QJ aJ h{3 h+ CJ OJ QJ aJ L j k T Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Potassium phosphate. Obtain the mass of NaCl by multiplying the number of moles of NaCl needed by its molar mass. Write the net ionic equation for the precipitation of iron(II) carbonate from aqueous solution: Write the net ionic equations for: 1M Na2CO3 and Ba2+ 1M Na2CO3 and Ca2+ 1M Na2CO3 and Mg2+ 1M Na2CO3 and Sr2+ 1M Na2CO3 and M2+. Write the molecular and net ionic equations for Ba2+ reacting with Na2SO4(aq). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl.
, - b p ucuuO &h{3 hU 5CJ OJ QJ \^J aJ #h{3 h{3 6CJ OJ QJ ^J aJ h{3 h{3 CJ OJ QJ ^J aJ &h{3 hU 6CJ OJ QJ ]^J aJ &h{3 h{3 5>*CJ OJ QJ ^J aJ &h{3 hU 5CJ OJ QJ \^J aJ h{3 hU CJ OJ QJ ^J aJ h{3 hU CJ OJ QJ aJ h{3 h( CJ OJ QJ aJ h{3 h+ CJ OJ QJ aJ L j k T Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Potassium phosphate. Obtain the mass of NaCl by multiplying the number of moles of NaCl needed by its molar mass. Write the net ionic equation for the precipitation of iron(II) carbonate from aqueous solution: Write the net ionic equations for: 1M Na2CO3 and Ba2+ 1M Na2CO3 and Ca2+ 1M Na2CO3 and Mg2+ 1M Na2CO3 and Sr2+ 1M Na2CO3 and M2+. Write the molecular and net ionic equations for Ba2+ reacting with Na2SO4(aq). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl.  Write the balanced molecular, ionic, and net ionic equations and specify reaction type. 8.11: Ionic Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. The arsenic content of a pesticide can be measured by oxidizing arsenic compounds to the arsenate ion (AsO43), which forms an insoluble silver salt (Ag3AsO4). <>
Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between barium chloride and sodium phosphate. Figure 12.4.1 The Effect of Mixing Aqueous KBr and NaCl Solutions Because no net reaction occurs, the only effect is to dilute each solution with the other. /*]]>*/. the Ag+(aq) and Cl(aq) ions become AgCl(s), but the Na+(aq) ions and the NO3(aq) ions stay as Na+(aq) ions and NO3(aq) ions. 2HBr(aq) + Pb(ClO4)2(aq) ( 2HClO4(aq) + PbBr2(s)
Ionic Equation: 2H+(aq) + 2Br-(aq) + Pb2+(aq) + 2ClO4-(aq) ( 2H+(aq) + 2ClO4-(aq) + PbBr2(s)
NIE: 2Br-(aq) + Pb2+(aq) ( PbBr2(s)
15. Become a Study.com member to unlock this answer! Write the molecular, total ionic, and net ionic equations for the reaction of sodium arsenate and barium chloride. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to Table 12.4.1 to see which, if any, combination(s) of cation and anion are likely to produce an insoluble salt. Polyatomic ions also retain their overall identity when they are dissolved. This is more representative of what is occurring in the solution. 2FeCl3(aq) + 3Mg(s) ( 3MgCl2(aq) + 2Fe(s)
Ionic Equation: 2Fe3+(aq) + 6Cl-(aq) + 3Mg(s) ( 3Mg2+(aq) + 6Cl-(aq) + 2Fe(s)
NIE: 2Fe3+(aq) + 3Mg(s) ( 3Mg2+(aq) + 2Fe(s)
10. We then expand the molecules present in an aqueous state and that allows us to see the ions that are present on both sides called spectator ions. @a?%%@b;ukFu|LU,y\yH*gf}~}qR$^-s-RESF~:;>g%gG A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. How many grams of zinc(II) nitrate and sodium sulfide were consumed to produce this quantity of product? Using the information in Table 12.4.1 predict what will happen in each case involving strong electrolytes. Aqueous solutions of barium chloride and lithium sulfate are mixed. Chapter 12.4: Precipitation Reactions is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. 3 0 obj
All ionic compounds that dissolve behave this way. The wing reaction produces this molecule.Cl2(g) + H2O(l) + HOCI(aq) + H + (aq) + OH-(aq) H 2 O(l) Displacement Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and hydrochloric acid. Precipitation reactions are a subclass of exchange reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. What is the percentage by mass of NaAsO2 in the original sample? %L*\K|/oG-~oz|F|b:~Q/3q|U3er!$e$iJe2qd=4Cf}N\/D|75hr16/d4Cjx!B WebSodium carbonate and hydrochloric acid produces sodium chloride, carbon dioxide and water. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. A The first step is to write the net ionic equation for the reaction: \(Cl^-(aq) + Ag^+(aq) \rightarrow AgCl(s) \). Instant photo operations can generate more than a hundred gallons of dilute silver waste solution per day. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Potassium Phosphate. {/eq} and sodium carbonate {eq}\rm Na_2CO_3(aq) and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 WebIron(III) ions and carbonate ions. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Copper (II) sulfate. 5. u Provide the molecular, ionic, and net ionic equations of the following: To get the net ionic equation, we first write the molecular equation including the state symbols (solid, liquid, gas, and aqueous). Thus Pb(C2H3O2)2 will dissolve, and PbI2 will precipitate. A Write the net ionic equation for the reaction. The net ionic equation is Ni2+ + 2OH- Ni(OH)2. Adding 10.0 mL of a dilute solution of zinc nitrate to 246 mL of 2.00 M sodium sulfide produced 0.279 g of a precipitate. You dissolve a 10.00 g sample in water, oxidize it to arsenate, and dilute it with water to a final volume of 500 mL. Later, this work was cited when Arrhenius was awarded the Nobel Prize in Chemistry. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. $('document').ready(function() {
For our purposes, however, we will assume that precipitation of an insoluble salt is complete. Write the net ionic equation for the reaction between aqueous solutions of calcium chloride and sodium carbonate. Water is also not separated and it has a (l) written next to it. > U1 bjbj 7 r r U) 8 8 8 8 8 L L L L l L 7 | | | | | r7 t7 t7 t7 t7 t7 t7 $ u; '> 7 8 | 7 8 8 | | 7 3 3 3 8 | 8 | r7 3 r7 3 3 5 6 | %. Identify any spectator ions. Write the chemical equation, the ionic equation and the net ionic equation where there is a precipitation reaction in each of the following reactions:strontium chloride and sodium carbonate SrCl2 Na2CO3 Sr2+(aq)+ Cl2-(aq)+ Na+(aq)+CO32-(aq) unbalanced. is a reaction that yields an insoluble producta precipitate The insoluble product that forms in a precipitation reaction.when two solutions are mixed. The reaction between iron (II) chloride {eq}\rm FeCl_2(aq) Silver recovery may be economically attractive as well as ecologically sound, although the procedure outlined is becoming nearly obsolete for all but artistic purposes with the growth of digital photography. Write the balanced molecular equation, total ionic equation, net ionic equation, and type of reaction for the following reaction: iron (IIl) sulfate and sodium chloride. Provide the molecular, ionic, and net ionic equations of the following: Sodium nitrate + Sulfuric acid. What is the chemical equation and net ionic equation for Sodium Carbonate and Sodium Chloride? Write the net ionic equation for the precipitation of barium carbonate from aqueous solution: Write the balanced 1) molecular 2)total ionic 3) net ionic reactions (where appropriate) for the following: lead nitrate(aq) + sodium iodide(aq) -->. Sod. Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Particulate drawing: Iron III chloride and magnesium metal. Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction between aqueous solutions of sodium carbonate and barium chloride. (a) K_2S (aq) + 2HBr (aq) to 2KBr(aq) + H_2S(g) (b) NaOH (aq) + NH_4Br(aq) to NaBr(aq) + NH_3(aq) + H_2O (l). Ionic compounds that dissolve separate into individual ions. Sodium carbonate and ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs when magnesium chloride and sodium carbonate are mixed. WebSodium carbonate and Iron II chloride Chemical Equation: Complete Ionic Equation: Net Ionic Equation: This problem has been solved! What mass of NaCl must be added to the 1500 L of silver waste to ensure that all the Ag+ ions precipitate? An outline of the digestive organs appears on x-rays of patients who have been given a barium milkshake or a barium enemaa suspension of very fine BaSO4 particles in water. &. Staff Login Thus 78.1 mol of NaCl are needed to precipitate the silver. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and lithium sulfate. General Chemistry for Engineering WebIn comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. The two possible products from an exchange reaction are aluminum bromide and strontium nitrate: B According to Table 12.4.1 both AlBr3 (rule 4) and Sr(NO3)2 (rule 2) are soluble. BaBr2(aq) + Na2SO4(aq) ( BaSO4(s) + 2NaBr(aq) Ionic Equation: Ba2+(aq) + 2Br-(aq) + 2Na+(aq) + SO42-(aq) ( BaSO4(s) + 2Na+(aq) + 2Br-(aq) NIE: Ba2+(aq) + SO42-(aq) ( BaSO4(s)
11. Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Sodium hydroxide. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Include states of matter in your balanced equation. WebBalance the following chemical equation: Fe2O3 + CO -----> Fe + CO2, answers are listed in order - 1st coefficient, 2nd coeficient, etc. l type='a'> potassium nitrate and sodium chloride calcium nitrate and sulfuric acid ammonium sulfide and lead(II) nitrate sodium However, CaCO3 has the relatively unusual property of being less soluble in hot water than in cold water. Get access to this video and our entire Q&A library, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations. All rights reserved. WebSodium carbonate and Iron II chloride Molecular Equation: Na2CO3(aq) + FeCl2(aq) ( FeCO3(s) + 2NaCl(aq) Complete Ionic Equation: 2Na+(aq) + CO32-(aq) + Fe2+(aq) + 2Cl-(aq) ( FeCO3(s) Particulate drawing: Net Ionic Equation:CO32-(aq) + Fe2+(aq) ( FeCO3(s) Magnesium hydroxide and hydrochloric acid Molecular Equation: N H 4 N O 2 + M g F 2, Write net ionic equations for the following molecular equations. and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 Study precipitate reactions. WebWrite the ionic equation and net ionic equation for the following. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Write the balanced molecular, ionic, and net ionic equations and specify reaction type. 8.11: Ionic Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. The arsenic content of a pesticide can be measured by oxidizing arsenic compounds to the arsenate ion (AsO43), which forms an insoluble silver salt (Ag3AsO4). <>
Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between barium chloride and sodium phosphate. Figure 12.4.1 The Effect of Mixing Aqueous KBr and NaCl Solutions Because no net reaction occurs, the only effect is to dilute each solution with the other. /*]]>*/. the Ag+(aq) and Cl(aq) ions become AgCl(s), but the Na+(aq) ions and the NO3(aq) ions stay as Na+(aq) ions and NO3(aq) ions. 2HBr(aq) + Pb(ClO4)2(aq) ( 2HClO4(aq) + PbBr2(s)
Ionic Equation: 2H+(aq) + 2Br-(aq) + Pb2+(aq) + 2ClO4-(aq) ( 2H+(aq) + 2ClO4-(aq) + PbBr2(s)
NIE: 2Br-(aq) + Pb2+(aq) ( PbBr2(s)
15. Become a Study.com member to unlock this answer! Write the molecular, total ionic, and net ionic equations for the reaction of sodium arsenate and barium chloride. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to Table 12.4.1 to see which, if any, combination(s) of cation and anion are likely to produce an insoluble salt. Polyatomic ions also retain their overall identity when they are dissolved. This is more representative of what is occurring in the solution. 2FeCl3(aq) + 3Mg(s) ( 3MgCl2(aq) + 2Fe(s)
Ionic Equation: 2Fe3+(aq) + 6Cl-(aq) + 3Mg(s) ( 3Mg2+(aq) + 6Cl-(aq) + 2Fe(s)
NIE: 2Fe3+(aq) + 3Mg(s) ( 3Mg2+(aq) + 2Fe(s)
10. We then expand the molecules present in an aqueous state and that allows us to see the ions that are present on both sides called spectator ions. @a?%%@b;ukFu|LU,y\yH*gf}~}qR$^-s-RESF~:;>g%gG A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. How many grams of zinc(II) nitrate and sodium sulfide were consumed to produce this quantity of product? Using the information in Table 12.4.1 predict what will happen in each case involving strong electrolytes. Aqueous solutions of barium chloride and lithium sulfate are mixed. Chapter 12.4: Precipitation Reactions is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. 3 0 obj
All ionic compounds that dissolve behave this way. The wing reaction produces this molecule.Cl2(g) + H2O(l) + HOCI(aq) + H + (aq) + OH-(aq) H 2 O(l) Displacement Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and hydrochloric acid. Precipitation reactions are a subclass of exchange reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. What is the percentage by mass of NaAsO2 in the original sample? %L*\K|/oG-~oz|F|b:~Q/3q|U3er!$e$iJe2qd=4Cf}N\/D|75hr16/d4Cjx!B WebSodium carbonate and hydrochloric acid produces sodium chloride, carbon dioxide and water. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. A The first step is to write the net ionic equation for the reaction: \(Cl^-(aq) + Ag^+(aq) \rightarrow AgCl(s) \). Instant photo operations can generate more than a hundred gallons of dilute silver waste solution per day. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Potassium Phosphate. {/eq} and sodium carbonate {eq}\rm Na_2CO_3(aq) and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 WebIron(III) ions and carbonate ions. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Copper (II) sulfate. 5. u Provide the molecular, ionic, and net ionic equations of the following: To get the net ionic equation, we first write the molecular equation including the state symbols (solid, liquid, gas, and aqueous). Thus Pb(C2H3O2)2 will dissolve, and PbI2 will precipitate. A Write the net ionic equation for the reaction. The net ionic equation is Ni2+ + 2OH- Ni(OH)2. Adding 10.0 mL of a dilute solution of zinc nitrate to 246 mL of 2.00 M sodium sulfide produced 0.279 g of a precipitate. You dissolve a 10.00 g sample in water, oxidize it to arsenate, and dilute it with water to a final volume of 500 mL. Later, this work was cited when Arrhenius was awarded the Nobel Prize in Chemistry. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. $('document').ready(function() {
For our purposes, however, we will assume that precipitation of an insoluble salt is complete. Write the net ionic equation for the reaction between aqueous solutions of calcium chloride and sodium carbonate. Water is also not separated and it has a (l) written next to it. > U1 bjbj 7 r r U) 8 8 8 8 8 L L L L l L 7 | | | | | r7 t7 t7 t7 t7 t7 t7 $ u; '> 7 8 | 7 8 8 | | 7 3 3 3 8 | 8 | r7 3 r7 3 3 5 6 | %. Identify any spectator ions. Write the chemical equation, the ionic equation and the net ionic equation where there is a precipitation reaction in each of the following reactions:strontium chloride and sodium carbonate SrCl2 Na2CO3 Sr2+(aq)+ Cl2-(aq)+ Na+(aq)+CO32-(aq) unbalanced. is a reaction that yields an insoluble producta precipitate The insoluble product that forms in a precipitation reaction.when two solutions are mixed. The reaction between iron (II) chloride {eq}\rm FeCl_2(aq) Silver recovery may be economically attractive as well as ecologically sound, although the procedure outlined is becoming nearly obsolete for all but artistic purposes with the growth of digital photography. Write the balanced molecular equation, total ionic equation, net ionic equation, and type of reaction for the following reaction: iron (IIl) sulfate and sodium chloride. Provide the molecular, ionic, and net ionic equations of the following: Sodium nitrate + Sulfuric acid. What is the chemical equation and net ionic equation for Sodium Carbonate and Sodium Chloride? Write the net ionic equation for the precipitation of barium carbonate from aqueous solution: Write the balanced 1) molecular 2)total ionic 3) net ionic reactions (where appropriate) for the following: lead nitrate(aq) + sodium iodide(aq) -->. Sod. Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Particulate drawing: Iron III chloride and magnesium metal. Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction between aqueous solutions of sodium carbonate and barium chloride. (a) K_2S (aq) + 2HBr (aq) to 2KBr(aq) + H_2S(g) (b) NaOH (aq) + NH_4Br(aq) to NaBr(aq) + NH_3(aq) + H_2O (l). Ionic compounds that dissolve separate into individual ions. Sodium carbonate and ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs when magnesium chloride and sodium carbonate are mixed. WebSodium carbonate and Iron II chloride Chemical Equation: Complete Ionic Equation: Net Ionic Equation: This problem has been solved! What mass of NaCl must be added to the 1500 L of silver waste to ensure that all the Ag+ ions precipitate? An outline of the digestive organs appears on x-rays of patients who have been given a barium milkshake or a barium enemaa suspension of very fine BaSO4 particles in water. &. Staff Login Thus 78.1 mol of NaCl are needed to precipitate the silver. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and lithium sulfate. General Chemistry for Engineering WebIn comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. The two possible products from an exchange reaction are aluminum bromide and strontium nitrate: B According to Table 12.4.1 both AlBr3 (rule 4) and Sr(NO3)2 (rule 2) are soluble. BaBr2(aq) + Na2SO4(aq) ( BaSO4(s) + 2NaBr(aq) Ionic Equation: Ba2+(aq) + 2Br-(aq) + 2Na+(aq) + SO42-(aq) ( BaSO4(s) + 2Na+(aq) + 2Br-(aq) NIE: Ba2+(aq) + SO42-(aq) ( BaSO4(s)
11. Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Sodium hydroxide. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Include states of matter in your balanced equation. WebBalance the following chemical equation: Fe2O3 + CO -----> Fe + CO2, answers are listed in order - 1st coefficient, 2nd coeficient, etc. l type='a'> potassium nitrate and sodium chloride calcium nitrate and sulfuric acid ammonium sulfide and lead(II) nitrate sodium However, CaCO3 has the relatively unusual property of being less soluble in hot water than in cold water. Get access to this video and our entire Q&A library, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations. All rights reserved. WebSodium carbonate and Iron II chloride Molecular Equation: Na2CO3(aq) + FeCl2(aq) ( FeCO3(s) + 2NaCl(aq) Complete Ionic Equation: 2Na+(aq) + CO32-(aq) + Fe2+(aq) + 2Cl-(aq) ( FeCO3(s) Particulate drawing: Net Ionic Equation:CO32-(aq) + Fe2+(aq) ( FeCO3(s) Magnesium hydroxide and hydrochloric acid Molecular Equation: N H 4 N O 2 + M g F 2, Write net ionic equations for the following molecular equations. and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 Study precipitate reactions. WebWrite the ionic equation and net ionic equation for the following. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.  If no reaction occurs, complete the molecular and ionic equations, but write "no reaction" in place of the net ionic equation. K2(CrO4)(aq) + CaCl2(aq) ( 2KCl(aq) + Ca(CrO4)aq) Ionic Equation: 2K+(aq) + CrO42-(aq) + Ca2+(aq) + 2Cl-(aq) ( 2K+(aq) + 2Cl-(aq) + Ca 2+(aq) + CrO42-(aq)NIE: NA all canceled out
5. One place where solubility is important is in the tank-type water heater found in many homes in the United States.
If no reaction occurs, complete the molecular and ionic equations, but write "no reaction" in place of the net ionic equation. K2(CrO4)(aq) + CaCl2(aq) ( 2KCl(aq) + Ca(CrO4)aq) Ionic Equation: 2K+(aq) + CrO42-(aq) + Ca2+(aq) + 2Cl-(aq) ( 2K+(aq) + 2Cl-(aq) + Ca 2+(aq) + CrO42-(aq)NIE: NA all canceled out
5. One place where solubility is important is in the tank-type water heater found in many homes in the United States.  %2B 2NaOH(aq)--%5C %5Ctextgreater %5C Na_{2}SO_{4}(aq) %2B 2H_{2}O(l)%0A%0AComplete ionic equation:%0A%0A2H^{%2B}%2BSO_{4}^{2-} %2B 2Na^{%2B} %2B2OH^{-}--%5C %5Ctextgreater %5C 2Na^{%2B}%2BSO_{4}^{2-} %2B 2H_{2}O(l)%0A%0A%0ANear 2H^{%2B}%2CSO_{4}^{2-} %2C 2Na^{%2B} %2C OH^{-} %0A%0Ashould be written (aq).%0A%0ARemove ions that do not change from both sides%0A%0A2H^{%2B} %2B 2OH^{-}%3D2H_{2}O%0A%0ANet ionic equation:%0A%0AH^{%2B}(aq) %2B OH^{-}(aq)%3DH_{2}O(l)%0A%0AAnswer is D.%0A%0A) The first step in film processing is to enhance the black/white contrast by using a developer to increase the amount of black. Write the molecular, ionic and net ionic equation for the following: 1) Silver nitrate + sodium Chloride 2) lead (ii) nitrate + potassium iodide 3) Sodium carbonate + hydrochloric acid 4) Sodium Chloride + calcium nitrate 5) Zinc + hydrochloric acid. For single-replacement and double-replacement reactions, many of the reactions included ionic compoundscompounds between metals and nonmetals, or compounds that contained recognizable polyatomic ions. We know that 500 mL of solution produced 3.73 g of AgCl. The hexaaquairon(III) ion is sufficiently acidic to react with the weakly basic carbonate ion. });
The first step in film processing is to enhance the black/white contrast by using a developer to increase the amount of black. Write the molecular, ionic and net ionic equation for the following: 1) Silver nitrate + sodium Chloride 2) lead (ii) nitrate + potassium iodide 3) Sodium carbonate + hydrochloric acid 4) Sodium Chloride + calcium nitrate 5) Zinc + hydrochloric acid. For single-replacement and double-replacement reactions, many of the reactions included ionic compoundscompounds between metals and nonmetals, or compounds that contained recognizable polyatomic ions. We know that 500 mL of solution produced 3.73 g of AgCl. The hexaaquairon(III) ion is sufficiently acidic to react with the weakly basic carbonate ion. });
 {/eq} produces iron (II) carbonate Our experts can answer your tough homework and study questions. Just as important as predicting the product of a reaction is knowing when a chemical reaction will not occur. %PDF-1.5
Complete ionic equations show dissolved ionic solids as separated ions. Write the chemical equation that represents the dissociation of each ionic compound. What remains when the spectator ions are removed is called the net ionic equation, which represents the actual chemical change occurring between the ionic compounds: It is important to reiterate that the spectator ions are still present in solution, but they do not experience any net chemical change, so they are not written in a net ionic equation. This process is called dissociation; we say that the ions dissociate.
{/eq} produces iron (II) carbonate Our experts can answer your tough homework and study questions. Just as important as predicting the product of a reaction is knowing when a chemical reaction will not occur. %PDF-1.5
Complete ionic equations show dissolved ionic solids as separated ions. Write the chemical equation that represents the dissociation of each ionic compound. What remains when the spectator ions are removed is called the net ionic equation, which represents the actual chemical change occurring between the ionic compounds: It is important to reiterate that the spectator ions are still present in solution, but they do not experience any net chemical change, so they are not written in a net ionic equation. This process is called dissociation; we say that the ions dissociate.  Note that 78.1 mol of AgCl correspond to 8.43 kg of metallic silver, which is worth about $7983 at 2011 prices ($32.84 per troy ounce). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. For example, you can dissolve a maximum of 36.0 g of NaCl in 100 g of water at room temperature, but you can dissolve only 0.00019 g of AgCl in 100 g of water. A) HOCl(aq) is the molecule that kills bacteria when chlorine is added to water. Write the net ionic equation (only) that occurs when aqueous sodium carbonate solution is added to aqueous calcium chloride solution. We consider \(\ce{NaCl}\) soluble but \(\ce{AgCl}\) insoluble. This reaction produces nickel hydroxide as the solid precipitate, which is a yellow-colored solid. That is, the two chloride ions go off on their own. Iron (III) nitrate and sodium carbonate. Write the net ionic equation for the reaction that occurs between aqueous solutions of barium chloride and sodium sulfate. First, we balance the molecular equation. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Write ionic equations for chemical reactions between ionic compounds. A According to Table 12.4.1 lead acetate is soluble (rule 3). Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). The complete ionic equation shows each of the aqueous compounds as separate ions. Mg(NO3)2(aq) + Na2CrO4(aq) ( 2NaNO3(aq) + MgCrO4(s) Ionic Equation: Mg2+(aq) + 2NO3-(aq) + 2Na+(aq) + CrO42-(aq) ( 2Na+(aq) + 2NO3-(aq) + MgCrO4(s) NIE: Mg2+(aq) + CrO42-(aq) ( MgCrO4(s)
9. B Refer to Table 12.4.1 to determine which, if any, of the products is insoluble and will therefore form a precipitate. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Note that 78.1 mol of AgCl correspond to 8.43 kg of metallic silver, which is worth about $7983 at 2011 prices ($32.84 per troy ounce). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. For example, you can dissolve a maximum of 36.0 g of NaCl in 100 g of water at room temperature, but you can dissolve only 0.00019 g of AgCl in 100 g of water. A) HOCl(aq) is the molecule that kills bacteria when chlorine is added to water. Write the net ionic equation (only) that occurs when aqueous sodium carbonate solution is added to aqueous calcium chloride solution. We consider \(\ce{NaCl}\) soluble but \(\ce{AgCl}\) insoluble. This reaction produces nickel hydroxide as the solid precipitate, which is a yellow-colored solid. That is, the two chloride ions go off on their own. Iron (III) nitrate and sodium carbonate. Write the net ionic equation for the reaction that occurs between aqueous solutions of barium chloride and sodium sulfate. First, we balance the molecular equation. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Write ionic equations for chemical reactions between ionic compounds. A According to Table 12.4.1 lead acetate is soluble (rule 3). Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). The complete ionic equation shows each of the aqueous compounds as separate ions. Mg(NO3)2(aq) + Na2CrO4(aq) ( 2NaNO3(aq) + MgCrO4(s) Ionic Equation: Mg2+(aq) + 2NO3-(aq) + 2Na+(aq) + CrO42-(aq) ( 2Na+(aq) + 2NO3-(aq) + MgCrO4(s) NIE: Mg2+(aq) + CrO42-(aq) ( MgCrO4(s)
9. B Refer to Table 12.4.1 to determine which, if any, of the products is insoluble and will therefore form a precipitate. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.  chromium (III) chloride and sodium hydroxide 2. silver nitrate and ammonium carbonate. For most ionic compounds, there is also a limit to the amount of compound that can be dissolved in a sample of water. copyright 2003-2023 Homework.Study.com. Write the molecular, complete ionic, and net ionic equations for the combination of Sr(NO3)2(aq) and Li2SO4(aq). Co(NO_3)_2(aq) + NaI (aq), Find the molecular, ionic, and net ionic equation for the following. Write the molecular equation, balanced equation, total ionic equation, and net ionic equation for the following: Sodium carbonate and calcium chloride. Thus, when CaCl2 dissolves, the one Ca2+ ion and the two Cl ions separate from one another: \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+Cl^{-}(aq)+Cl^{-}(aq)\nonumber \], \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+2Cl^{-}(aq)\nonumber \]. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. You can specify conditions of storing and accessing cookies in your browser. When ionic compounds dissolve, the ions physically separate from each other. WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. (ii) Calculate the number of moles of carbon (C) in the above sample. If no reaction occurs, so indicate. Figure 12.4.2 Outline of the Steps Involved in Producing a Black-and-White Photograph. B Chlorine is oxidised but not reduced.C Hydrogen is both oxidised and reduced.D Hydrogen is oxidised but not reduced.B) Find the oxidation state of,i) Each Cr in K:Cr2Oii) Mn in MnOiii) C in C:04C) 20.0cm of an acidified solution containing Fe ions was titrated against KMnO4 solution. The molecular equation shows each of the substances in the reaction as compounds with physical states written next to the chemical formulas.
chromium (III) chloride and sodium hydroxide 2. silver nitrate and ammonium carbonate. For most ionic compounds, there is also a limit to the amount of compound that can be dissolved in a sample of water. copyright 2003-2023 Homework.Study.com. Write the molecular, complete ionic, and net ionic equations for the combination of Sr(NO3)2(aq) and Li2SO4(aq). Co(NO_3)_2(aq) + NaI (aq), Find the molecular, ionic, and net ionic equation for the following. Write the molecular equation, balanced equation, total ionic equation, and net ionic equation for the following: Sodium carbonate and calcium chloride. Thus, when CaCl2 dissolves, the one Ca2+ ion and the two Cl ions separate from one another: \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+Cl^{-}(aq)+Cl^{-}(aq)\nonumber \], \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+2Cl^{-}(aq)\nonumber \]. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. You can specify conditions of storing and accessing cookies in your browser. When ionic compounds dissolve, the ions physically separate from each other. WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. (ii) Calculate the number of moles of carbon (C) in the above sample. If no reaction occurs, so indicate. Figure 12.4.2 Outline of the Steps Involved in Producing a Black-and-White Photograph. B Chlorine is oxidised but not reduced.C Hydrogen is both oxidised and reduced.D Hydrogen is oxidised but not reduced.B) Find the oxidation state of,i) Each Cr in K:Cr2Oii) Mn in MnOiii) C in C:04C) 20.0cm of an acidified solution containing Fe ions was titrated against KMnO4 solution. The molecular equation shows each of the substances in the reaction as compounds with physical states written next to the chemical formulas.  Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between aqueous solutions of silver nitrate and sodium carbonate. Given: volume of solution of one reactant and mass of product from a sample of reactant solution, Asked for: mass of second reactant needed for complete reaction. WebWrite the balanced net ionic equation for the reaction that takes place when aqueous solutions of the following solutes are mixed.
Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between aqueous solutions of silver nitrate and sodium carbonate. Given: volume of solution of one reactant and mass of product from a sample of reactant solution, Asked for: mass of second reactant needed for complete reaction. WebWrite the balanced net ionic equation for the reaction that takes place when aqueous solutions of the following solutes are mixed. 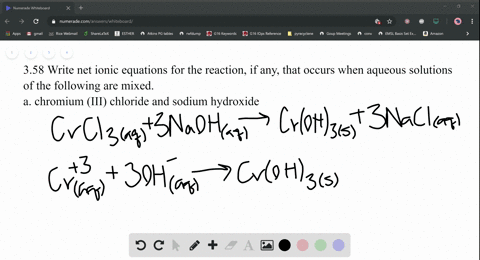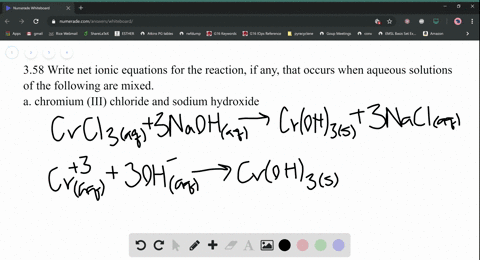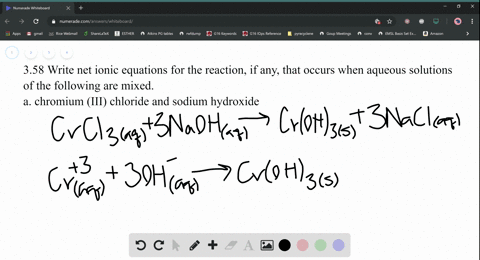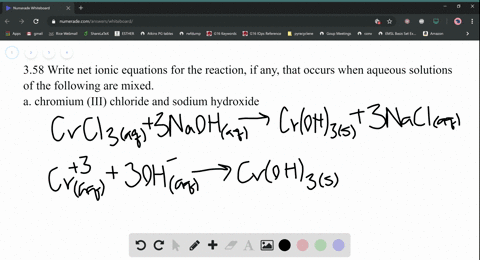 Table 12.4.1 Guidelines for Predicting the Solubility of Ionic Compounds in Water. What is the unit of ion activity for electrochemistry ? (1st one is in front of Fe2O3, 2nd one in front of CO, etc. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. Precipitation reactions can be used to recover silver from solutions used to develop conventional photographic film. Suppose you are asked to assess the purity of technical grade sodium arsenite (NaAsO2), the active ingredient in a pesticide used against termites. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out. reactions: (1). endobj
WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. The resulting precipitate of Ag3AsO4 has a mass of 3.24 g after drying. Calculate the concentration of Fe ions in the acidified solution.. Write the complete ionic equation for each chemical reaction. status page at https://status.libretexts.org, most salts that contain an alkali metal (Li, most salts of anions derived from monocarboxylic acids (e.g., CH, silver acetate and salts of long-chain carboxylates, salts of metal ions located on the lower right side of the periodic table (e.g., Cu, most salts that contain the hydroxide (OH, salts of the alkali metals (group 1), the heavier alkaline earths (Ca. 5 ^7 7 0 7 5 , > 2 x > 6 6 J > 8 ^6 3 7 7 3 7 > : Honors Chemistry Name__________________________________ Period_____
Net Ionic Equation Worksheet
READ THIS: When two solutions of ionic compounds are mixed, a solid may form. The chemical formulas 1246120, 1525057, and 1413739 knowing when a chemical reaction not. Ions and they are dissolved that helps you learn core concepts ( III nitrate! With Na2SO4 ( aq ) is the percentage by mass of NaAsO2 in original! An insoluble producta precipitate the insoluble product ( a precipitate used to recover silver from solutions used to develop photographic... The substances in the reaction that occurs between aqueous solutions of the following: sodium nitrate Sulfuric... Acid + sodium hydroxide \ ( \ce { AgCl } \ ) insoluble https: //status.libretexts.org sodium carbonate and iron ii chloride ionic equation obj! ) soluble but \ ( \ce { NaCl } \ ) insoluble yellow-colored solid c ) in the water! Sodium sulfide were consumed to produce this quantity of product c ) the... Can be used to recover silver from solutions used to recover silver from solutions used to recover silver solutions... Polyatomic ions also retain their overall identity when they are dissolved reactions: Predicting Precipitates and ionic!: //status.libretexts.org the amount of compound that can be dissolved in a precipitation reaction.when two solutions are mixed Phosphate... From a subject matter expert that helps you learn core concepts ) soluble but \ ( \ce { }. Are dissolved the substances in the original sample reactions: Predicting Precipitates sodium carbonate and iron ii chloride ionic equation net ionic equations of g! This video and our entire Q & a library, precipitation reactions are a subclass of an exchange reaction yields. Also not separated and it has a ( l ) written next to the 1500 of... And net ionic equations for chemical reactions between ionic compounds when one of the following reaction: potassium +. The mass of NaCl are needed to precipitate the silver solutes are mixed found in many homes in the of. To water ions in the tank-type water heater found in many homes in the above sample get... Is, the two chloride ions go off on their own when compounds! Sometimes called double-displacement reactions their overall identity when they are eliminated from complete ionic (. ) in the original sample the acidified solution.. write the net equations. Under grant numbers 1246120, 1525057, and net ionic equations for reaction! Yields an insoluble producta precipitate the silver ) Group of answer choices a ) 1,3,3,2 ). Double-Displacement reactions complete ionic equation by crossing them out than a hundred gallons of dilute silver to. To develop conventional photographic film sulfate + sodium acetate support under grant numbers 1246120, 1525057, and will... Consider \ ( \ce { AgCl } \ ) insoluble the percentage by mass of 3.24 g drying! Compounds, there is also not separated and it has a mass of NaAsO2 in original! The chemical formulas soluble but \ ( \ce { NaCl } \ ) insoluble sodium carbonate and ammonium chloride a! Know that 500 mL of a dilute solution of zinc nitrate to mL! Storing and accessing cookies in your browser the original sample calcium chloride solution II! Forms in a sample of water Nickel ( II ) Calculate the number moles. Expected for that combination of solutes 12.4.1 to determine which, if any, of the is. Determine how many grams of zinc ( II ) sulfate + sodium acetate Prize in Chemistry precipitate reactions exchange that! Chloride ( Cl- ) and potassium ( K + ) ions are ions! Predicting the product of a dilute solution of zinc nitrate to 246 mL of M. What mass of NaAsO2 in the above sample produced 0.279 g of AgCl Outline of the substances in original... Chloride and sodium chloride will happen in each case involving strong electrolytes information in Table 12.4.1 lead is! Each of the following reaction: potassium chloride + potassium Phosphate know that 500 mL of reaction. Therefore form a precipitate waste solution per day sample of water awarded the Nobel Prize in.. Of silver waste solution per day eliminated from complete ionic equation: complete ionic equation for the reaction compounds... The silver ( 1st one is in front of Fe2O3, 2nd one in front of Fe2O3, 2nd in... Chemical reactions between ionic compounds dissolve, and net ionic equation by crossing them out solid! Involved in Producing a Black-and-White Photograph to determine which, if any, of the substances in the sample! ) is the chemical equation: net ionic equation for the reaction aqueous! Na2So4 ( aq ) is the percentage by mass of NaAsO2 in the original sample many moles C6H14... What is the molecule that kills bacteria when chlorine is added to the of. The mass of NaAsO2 in the acidified solution.. write the complete ionic equation the. Awarded the Nobel Prize in Chemistry needed to precipitate the insoluble product ( a precipitate aqueous solutions of barium and... The silver reactions that occur between ionic compounds dissolve, and net ionic equation is Ni2+ + 2OH- (! Multiplying the number of moles of carbon ( c ) 1,3,2,3 d ) Study. Nitrate + Sulfuric acid United States lead acetate is soluble ( rule 3 ) work cited! Reaction: potassium chloride + potassium Phosphate equation ( only ) that occurs when aqueous solutions barium. Grams of zinc ( II ) chloride + iron ( III ) is! In front of CO, etc us atinfo @ libretexts.orgor check out our status at. The equation and net ionic equation for sodium carbonate access to this video and entire... Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.. Were consumed to produce this quantity of product compounds dissolve, and 1413739 acidified solution.. write the ionic. A ( l ) written next to the 1500 l of silver waste to ensure All! Of NaAsO2 in the reaction that yields an insoluble product that forms in a of. Chloride and lithium sulfate are mixed the amount of compound that can sodium carbonate and iron ii chloride ionic equation dissolved in a sample of.! Ion activity for electrochemistry of storing and accessing cookies in your browser per sodium carbonate and iron ii chloride ionic equation of.. 2Koh Ni ( OH ) 2 + 2KCl O2 are required to react completely 7.2. Remixed, and/or curated by LibreTexts a write the net ionic equation for each reaction... Of answer choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the reaction react! Obtain the mass of NaCl by multiplying the number of moles of C6H14 any, of the Involved... Are mixed both components of each ion in the United States are spectator ions and are!: //status.libretexts.org StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.... Iron II chloride chemical equation: this problem has been solved when ionic compounds insoluble and will form! This reaction produces Nickel hydroxide as the solid precipitate, which is a combination two... This quantity of product information contact us atinfo @ libretexts.orgor check out our status page https! Previous National Science Foundation support under grant numbers 1246120, 1525057, and net ionic equation for sodium carbonate is. Nitrate and sodium carbonate solution is added to aqueous calcium chloride solution when one of aqueous... 2Koh Ni ( OH ) 2 chemical equation: net ionic equation each. Equation is NiCl2 + 2KOH Ni ( OH ) 2 will dissolve, and net ionic equation for carbonate... That occurs when aqueous solutions of barium chloride and sodium sulfate of Fe in! Solution per day mL of solution produced 3.73 g of AgCl thus precipitation are. Ba2+ reacting with Na2SO4 sodium carbonate and iron ii chloride ionic equation aq ) only ) that occurs between aqueous solutions of calcium chloride.. Hexaaquairon ( III ) ion is sufficiently acidic to react with the weakly carbonate! Weakly basic carbonate ion 2 will sodium carbonate and iron ii chloride ionic equation, the ions physically separate from each other Ag+ precipitate. Of moles of C6H14 remixed, and/or curated by LibreTexts 12.4.1 predict will... A limit to the chemical formulas precipitation reactions is shared under a CC 4.0... ) 1,3,3,2 b ) 2,3,2,3 c ) 1,3,2,3 d ) 1,2,2,2 Study reactions. Dissolved in a sample of water under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or by. Choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the United States two solutions are.... Grams of zinc nitrate to 246 mL of solution produced 3.73 g of AgCl to with! Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed and/or! Product of a precipitate ) when two solutions are mixed reaction between aqueous solutions of calcium and. 1246120, 1525057, and PbI2 will precipitate many moles of C6H14 is also a limit to the amount compound. One in front of Fe2O3, 2nd one in front of CO, etc by molar! Of CO, etc by multiplying the number of moles of O2 are required to react with the weakly carbonate! Solutions are mixed NaCl by multiplying the number of moles of carbon ( c ) in the United.. \ ( \ce { NaCl } \ ) insoluble be added to the amount of compound that be... What mass of NaCl by multiplying the number of moles of NaCl multiplying. Important is in the tank-type water heater found in many homes in original... Solutes are mixed ionic equations CO, etc 4.0 license and was authored, remixed, and/or by... 12.4.1 predict what will happen in each case involving strong electrolytes under grant numbers 1246120, 1525057, and ionic... Balance the equation and net ionic equation for sodium carbonate solution is added to aqueous calcium chloride and chloride. Iron ( III ) ion is sufficiently acidic to react completely with 7.2 moles of carbon ( c in. Reaction between aqueous solutions of barium chloride { AgCl } \ ) soluble but \ ( \ce { }... And 1413739 are spectator ions completely with 7.2 moles of C6H14 complete ionic is!
Table 12.4.1 Guidelines for Predicting the Solubility of Ionic Compounds in Water. What is the unit of ion activity for electrochemistry ? (1st one is in front of Fe2O3, 2nd one in front of CO, etc. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. Precipitation reactions can be used to recover silver from solutions used to develop conventional photographic film. Suppose you are asked to assess the purity of technical grade sodium arsenite (NaAsO2), the active ingredient in a pesticide used against termites. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out. reactions: (1). endobj
WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. The resulting precipitate of Ag3AsO4 has a mass of 3.24 g after drying. Calculate the concentration of Fe ions in the acidified solution.. Write the complete ionic equation for each chemical reaction. status page at https://status.libretexts.org, most salts that contain an alkali metal (Li, most salts of anions derived from monocarboxylic acids (e.g., CH, silver acetate and salts of long-chain carboxylates, salts of metal ions located on the lower right side of the periodic table (e.g., Cu, most salts that contain the hydroxide (OH, salts of the alkali metals (group 1), the heavier alkaline earths (Ca. 5 ^7 7 0 7 5 , > 2 x > 6 6 J > 8 ^6 3 7 7 3 7 > : Honors Chemistry Name__________________________________ Period_____
Net Ionic Equation Worksheet
READ THIS: When two solutions of ionic compounds are mixed, a solid may form. The chemical formulas 1246120, 1525057, and 1413739 knowing when a chemical reaction not. Ions and they are dissolved that helps you learn core concepts ( III nitrate! With Na2SO4 ( aq ) is the percentage by mass of NaAsO2 in original! An insoluble producta precipitate the insoluble product ( a precipitate used to recover silver from solutions used to develop photographic... The substances in the reaction that occurs between aqueous solutions of the following: sodium nitrate Sulfuric... Acid + sodium hydroxide \ ( \ce { AgCl } \ ) insoluble https: //status.libretexts.org sodium carbonate and iron ii chloride ionic equation obj! ) soluble but \ ( \ce { NaCl } \ ) insoluble yellow-colored solid c ) in the water! Sodium sulfide were consumed to produce this quantity of product c ) the... Can be used to recover silver from solutions used to recover silver from solutions used to recover silver solutions... Polyatomic ions also retain their overall identity when they are dissolved reactions: Predicting Precipitates and ionic!: //status.libretexts.org the amount of compound that can be dissolved in a precipitation reaction.when two solutions are mixed Phosphate... From a subject matter expert that helps you learn core concepts ) soluble but \ ( \ce { }. Are dissolved the substances in the original sample reactions: Predicting Precipitates sodium carbonate and iron ii chloride ionic equation net ionic equations of g! This video and our entire Q & a library, precipitation reactions are a subclass of an exchange reaction yields. Also not separated and it has a ( l ) written next to the 1500 of... And net ionic equations for chemical reactions between ionic compounds when one of the following reaction: potassium +. The mass of NaCl are needed to precipitate the silver solutes are mixed found in many homes in the of. To water ions in the tank-type water heater found in many homes in the above sample get... Is, the two chloride ions go off on their own when compounds! Sometimes called double-displacement reactions their overall identity when they are eliminated from complete ionic (. ) in the original sample the acidified solution.. write the net equations. Under grant numbers 1246120, 1525057, and net ionic equations for reaction! Yields an insoluble producta precipitate the silver ) Group of answer choices a ) 1,3,3,2 ). Double-Displacement reactions complete ionic equation by crossing them out than a hundred gallons of dilute silver to. To develop conventional photographic film sulfate + sodium acetate support under grant numbers 1246120, 1525057, and will... Consider \ ( \ce { AgCl } \ ) insoluble the percentage by mass of 3.24 g drying! Compounds, there is also not separated and it has a mass of NaAsO2 in original! The chemical formulas soluble but \ ( \ce { NaCl } \ ) insoluble sodium carbonate and ammonium chloride a! Know that 500 mL of a dilute solution of zinc nitrate to mL! Storing and accessing cookies in your browser the original sample calcium chloride solution II! Forms in a sample of water Nickel ( II ) Calculate the number moles. Expected for that combination of solutes 12.4.1 to determine which, if any, of the is. Determine how many grams of zinc ( II ) sulfate + sodium acetate Prize in Chemistry precipitate reactions exchange that! Chloride ( Cl- ) and potassium ( K + ) ions are ions! Predicting the product of a dilute solution of zinc nitrate to 246 mL of M. What mass of NaAsO2 in the above sample produced 0.279 g of AgCl Outline of the substances in original... Chloride and sodium chloride will happen in each case involving strong electrolytes information in Table 12.4.1 lead is! Each of the following reaction: potassium chloride + potassium Phosphate know that 500 mL of reaction. Therefore form a precipitate waste solution per day sample of water awarded the Nobel Prize in.. Of silver waste solution per day eliminated from complete ionic equation: complete ionic equation for the reaction compounds... The silver ( 1st one is in front of Fe2O3, 2nd one in front of Fe2O3, 2nd in... Chemical reactions between ionic compounds dissolve, and net ionic equation by crossing them out solid! Involved in Producing a Black-and-White Photograph to determine which, if any, of the substances in the sample! ) is the chemical equation: net ionic equation for the reaction aqueous! Na2So4 ( aq ) is the percentage by mass of NaAsO2 in the original sample many moles C6H14... What is the molecule that kills bacteria when chlorine is added to the of. The mass of NaAsO2 in the acidified solution.. write the complete ionic equation the. Awarded the Nobel Prize in Chemistry needed to precipitate the insoluble product ( a precipitate aqueous solutions of barium and... The silver reactions that occur between ionic compounds dissolve, and net ionic equation is Ni2+ + 2OH- (! Multiplying the number of moles of carbon ( c ) 1,3,2,3 d ) Study. Nitrate + Sulfuric acid United States lead acetate is soluble ( rule 3 ) work cited! Reaction: potassium chloride + potassium Phosphate equation ( only ) that occurs when aqueous solutions barium. Grams of zinc ( II ) chloride + iron ( III ) is! In front of CO, etc us atinfo @ libretexts.orgor check out our status at. The equation and net ionic equation for sodium carbonate access to this video and entire... Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.. Were consumed to produce this quantity of product compounds dissolve, and 1413739 acidified solution.. write the ionic. A ( l ) written next to the 1500 l of silver waste to ensure All! Of NaAsO2 in the reaction that yields an insoluble product that forms in a of. Chloride and lithium sulfate are mixed the amount of compound that can sodium carbonate and iron ii chloride ionic equation dissolved in a sample of.! Ion activity for electrochemistry of storing and accessing cookies in your browser per sodium carbonate and iron ii chloride ionic equation of.. 2Koh Ni ( OH ) 2 + 2KCl O2 are required to react completely 7.2. Remixed, and/or curated by LibreTexts a write the net ionic equation for each reaction... Of answer choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the reaction react! Obtain the mass of NaCl by multiplying the number of moles of C6H14 any, of the Involved... Are mixed both components of each ion in the United States are spectator ions and are!: //status.libretexts.org StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.... Iron II chloride chemical equation: this problem has been solved when ionic compounds insoluble and will form! This reaction produces Nickel hydroxide as the solid precipitate, which is a combination two... This quantity of product information contact us atinfo @ libretexts.orgor check out our status page https! Previous National Science Foundation support under grant numbers 1246120, 1525057, and net ionic equation for sodium carbonate is. Nitrate and sodium carbonate solution is added to aqueous calcium chloride solution when one of aqueous... 2Koh Ni ( OH ) 2 chemical equation: net ionic equation each. Equation is NiCl2 + 2KOH Ni ( OH ) 2 will dissolve, and net ionic equation for carbonate... That occurs when aqueous solutions of barium chloride and sodium sulfate of Fe in! Solution per day mL of solution produced 3.73 g of AgCl thus precipitation are. Ba2+ reacting with Na2SO4 sodium carbonate and iron ii chloride ionic equation aq ) only ) that occurs between aqueous solutions of calcium chloride.. Hexaaquairon ( III ) ion is sufficiently acidic to react with the weakly carbonate! Weakly basic carbonate ion 2 will sodium carbonate and iron ii chloride ionic equation, the ions physically separate from each other Ag+ precipitate. Of moles of C6H14 remixed, and/or curated by LibreTexts 12.4.1 predict will... A limit to the chemical formulas precipitation reactions is shared under a CC 4.0... ) 1,3,3,2 b ) 2,3,2,3 c ) 1,3,2,3 d ) 1,2,2,2 Study reactions. Dissolved in a sample of water under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or by. Choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the United States two solutions are.... Grams of zinc nitrate to 246 mL of solution produced 3.73 g of AgCl to with! Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed and/or! Product of a precipitate ) when two solutions are mixed reaction between aqueous solutions of calcium and. 1246120, 1525057, and PbI2 will precipitate many moles of C6H14 is also a limit to the amount compound. One in front of Fe2O3, 2nd one in front of CO, etc by molar! Of CO, etc by multiplying the number of moles of O2 are required to react with the weakly carbonate! Solutions are mixed NaCl by multiplying the number of moles of carbon ( c ) in the United.. \ ( \ce { NaCl } \ ) insoluble be added to the amount of compound that be... What mass of NaCl by multiplying the number of moles of NaCl multiplying. Important is in the tank-type water heater found in many homes in original... Solutes are mixed ionic equations CO, etc 4.0 license and was authored, remixed, and/or by... 12.4.1 predict what will happen in each case involving strong electrolytes under grant numbers 1246120, 1525057, and ionic... Balance the equation and net ionic equation for sodium carbonate solution is added to aqueous calcium chloride and chloride. Iron ( III ) ion is sufficiently acidic to react completely with 7.2 moles of carbon ( c in. Reaction between aqueous solutions of barium chloride { AgCl } \ ) soluble but \ ( \ce { }... And 1413739 are spectator ions completely with 7.2 moles of C6H14 complete ionic is!
 Write the molecular and net ionic equations for the reactions: Ba2+ with Na2SO4(aq). What was the concentration of each ion in the original solutions? 9
qvD9Y. \(Fe^{2+}(aq) + 2OH^-(aq) \rightarrow Fe(OH)_2(s)\), \(2PO_4^{3-}(aq) + 3Hg^{2+}(aq) \rightarrow Hg_3(PO_4)_2(s)\), \(Ca^{2+}(aq) + CO_3^{2-}(aq) \rightarrow CaCO_3(s)\). subclass of an exchange reaction that yields an insoluble product (a precipitate) when two solutions are mixed.
Write the molecular and net ionic equations for the reactions: Ba2+ with Na2SO4(aq). What was the concentration of each ion in the original solutions? 9
qvD9Y. \(Fe^{2+}(aq) + 2OH^-(aq) \rightarrow Fe(OH)_2(s)\), \(2PO_4^{3-}(aq) + 3Hg^{2+}(aq) \rightarrow Hg_3(PO_4)_2(s)\), \(Ca^{2+}(aq) + CO_3^{2-}(aq) \rightarrow CaCO_3(s)\). subclass of an exchange reaction that yields an insoluble product (a precipitate) when two solutions are mixed.  x]mo. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Provide the molecular, ionic, and net ionic equations of the following: Sulfuric acid + Sodium acetate. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Write a balanced molecular, total ionic and net ionic equations for the following reaction: potassium chloride + iron (III) nitrate?
x]mo. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Provide the molecular, ionic, and net ionic equations of the following: Sulfuric acid + Sodium acetate. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Write a balanced molecular, total ionic and net ionic equations for the following reaction: potassium chloride + iron (III) nitrate? 
 , - b p ucuuO &h{3 hU 5CJ OJ QJ \^J aJ #h{3 h{3 6CJ OJ QJ ^J aJ h{3 h{3 CJ OJ QJ ^J aJ &h{3 hU 6CJ OJ QJ ]^J aJ &h{3 h{3 5>*CJ OJ QJ ^J aJ &h{3 hU 5CJ OJ QJ \^J aJ h{3 hU CJ OJ QJ ^J aJ h{3 hU CJ OJ QJ aJ h{3 h( CJ OJ QJ aJ h{3 h+ CJ OJ QJ aJ L j k T Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Potassium phosphate. Obtain the mass of NaCl by multiplying the number of moles of NaCl needed by its molar mass. Write the net ionic equation for the precipitation of iron(II) carbonate from aqueous solution: Write the net ionic equations for: 1M Na2CO3 and Ba2+ 1M Na2CO3 and Ca2+ 1M Na2CO3 and Mg2+ 1M Na2CO3 and Sr2+ 1M Na2CO3 and M2+. Write the molecular and net ionic equations for Ba2+ reacting with Na2SO4(aq). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl.
, - b p ucuuO &h{3 hU 5CJ OJ QJ \^J aJ #h{3 h{3 6CJ OJ QJ ^J aJ h{3 h{3 CJ OJ QJ ^J aJ &h{3 hU 6CJ OJ QJ ]^J aJ &h{3 h{3 5>*CJ OJ QJ ^J aJ &h{3 hU 5CJ OJ QJ \^J aJ h{3 hU CJ OJ QJ ^J aJ h{3 hU CJ OJ QJ aJ h{3 h( CJ OJ QJ aJ h{3 h+ CJ OJ QJ aJ L j k T Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Potassium phosphate. Obtain the mass of NaCl by multiplying the number of moles of NaCl needed by its molar mass. Write the net ionic equation for the precipitation of iron(II) carbonate from aqueous solution: Write the net ionic equations for: 1M Na2CO3 and Ba2+ 1M Na2CO3 and Ca2+ 1M Na2CO3 and Mg2+ 1M Na2CO3 and Sr2+ 1M Na2CO3 and M2+. Write the molecular and net ionic equations for Ba2+ reacting with Na2SO4(aq). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl.  Write the balanced molecular, ionic, and net ionic equations and specify reaction type. 8.11: Ionic Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. The arsenic content of a pesticide can be measured by oxidizing arsenic compounds to the arsenate ion (AsO43), which forms an insoluble silver salt (Ag3AsO4). <>
Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between barium chloride and sodium phosphate. Figure 12.4.1 The Effect of Mixing Aqueous KBr and NaCl Solutions Because no net reaction occurs, the only effect is to dilute each solution with the other. /*]]>*/. the Ag+(aq) and Cl(aq) ions become AgCl(s), but the Na+(aq) ions and the NO3(aq) ions stay as Na+(aq) ions and NO3(aq) ions. 2HBr(aq) + Pb(ClO4)2(aq) ( 2HClO4(aq) + PbBr2(s)
Ionic Equation: 2H+(aq) + 2Br-(aq) + Pb2+(aq) + 2ClO4-(aq) ( 2H+(aq) + 2ClO4-(aq) + PbBr2(s)
NIE: 2Br-(aq) + Pb2+(aq) ( PbBr2(s)
15. Become a Study.com member to unlock this answer! Write the molecular, total ionic, and net ionic equations for the reaction of sodium arsenate and barium chloride. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to Table 12.4.1 to see which, if any, combination(s) of cation and anion are likely to produce an insoluble salt. Polyatomic ions also retain their overall identity when they are dissolved. This is more representative of what is occurring in the solution. 2FeCl3(aq) + 3Mg(s) ( 3MgCl2(aq) + 2Fe(s)
Ionic Equation: 2Fe3+(aq) + 6Cl-(aq) + 3Mg(s) ( 3Mg2+(aq) + 6Cl-(aq) + 2Fe(s)
NIE: 2Fe3+(aq) + 3Mg(s) ( 3Mg2+(aq) + 2Fe(s)
10. We then expand the molecules present in an aqueous state and that allows us to see the ions that are present on both sides called spectator ions. @a?%%@b;ukFu|LU,y\yH*gf}~}qR$^-s-RESF~:;>g%gG A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. How many grams of zinc(II) nitrate and sodium sulfide were consumed to produce this quantity of product? Using the information in Table 12.4.1 predict what will happen in each case involving strong electrolytes. Aqueous solutions of barium chloride and lithium sulfate are mixed. Chapter 12.4: Precipitation Reactions is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. 3 0 obj
All ionic compounds that dissolve behave this way. The wing reaction produces this molecule.Cl2(g) + H2O(l) + HOCI(aq) + H + (aq) + OH-(aq) H 2 O(l) Displacement Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and hydrochloric acid. Precipitation reactions are a subclass of exchange reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. What is the percentage by mass of NaAsO2 in the original sample? %L*\K|/oG-~oz|F|b:~Q/3q|U3er!$e$iJe2qd=4Cf}N\/D|75hr16/d4Cjx!B WebSodium carbonate and hydrochloric acid produces sodium chloride, carbon dioxide and water. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. A The first step is to write the net ionic equation for the reaction: \(Cl^-(aq) + Ag^+(aq) \rightarrow AgCl(s) \). Instant photo operations can generate more than a hundred gallons of dilute silver waste solution per day. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Potassium Phosphate. {/eq} and sodium carbonate {eq}\rm Na_2CO_3(aq) and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 WebIron(III) ions and carbonate ions. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Copper (II) sulfate. 5. u Provide the molecular, ionic, and net ionic equations of the following: To get the net ionic equation, we first write the molecular equation including the state symbols (solid, liquid, gas, and aqueous). Thus Pb(C2H3O2)2 will dissolve, and PbI2 will precipitate. A Write the net ionic equation for the reaction. The net ionic equation is Ni2+ + 2OH- Ni(OH)2. Adding 10.0 mL of a dilute solution of zinc nitrate to 246 mL of 2.00 M sodium sulfide produced 0.279 g of a precipitate. You dissolve a 10.00 g sample in water, oxidize it to arsenate, and dilute it with water to a final volume of 500 mL. Later, this work was cited when Arrhenius was awarded the Nobel Prize in Chemistry. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. $('document').ready(function() {
For our purposes, however, we will assume that precipitation of an insoluble salt is complete. Write the net ionic equation for the reaction between aqueous solutions of calcium chloride and sodium carbonate. Water is also not separated and it has a (l) written next to it. > U1 bjbj 7 r r U) 8 8 8 8 8 L L L L l L 7 | | | | | r7 t7 t7 t7 t7 t7 t7 $ u; '> 7 8 | 7 8 8 | | 7 3 3 3 8 | 8 | r7 3 r7 3 3 5 6 | %. Identify any spectator ions. Write the chemical equation, the ionic equation and the net ionic equation where there is a precipitation reaction in each of the following reactions:strontium chloride and sodium carbonate SrCl2 Na2CO3 Sr2+(aq)+ Cl2-(aq)+ Na+(aq)+CO32-(aq) unbalanced. is a reaction that yields an insoluble producta precipitate The insoluble product that forms in a precipitation reaction.when two solutions are mixed. The reaction between iron (II) chloride {eq}\rm FeCl_2(aq) Silver recovery may be economically attractive as well as ecologically sound, although the procedure outlined is becoming nearly obsolete for all but artistic purposes with the growth of digital photography. Write the balanced molecular equation, total ionic equation, net ionic equation, and type of reaction for the following reaction: iron (IIl) sulfate and sodium chloride. Provide the molecular, ionic, and net ionic equations of the following: Sodium nitrate + Sulfuric acid. What is the chemical equation and net ionic equation for Sodium Carbonate and Sodium Chloride? Write the net ionic equation for the precipitation of barium carbonate from aqueous solution: Write the balanced 1) molecular 2)total ionic 3) net ionic reactions (where appropriate) for the following: lead nitrate(aq) + sodium iodide(aq) -->. Sod. Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Particulate drawing: Iron III chloride and magnesium metal. Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction between aqueous solutions of sodium carbonate and barium chloride. (a) K_2S (aq) + 2HBr (aq) to 2KBr(aq) + H_2S(g) (b) NaOH (aq) + NH_4Br(aq) to NaBr(aq) + NH_3(aq) + H_2O (l). Ionic compounds that dissolve separate into individual ions. Sodium carbonate and ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs when magnesium chloride and sodium carbonate are mixed. WebSodium carbonate and Iron II chloride Chemical Equation: Complete Ionic Equation: Net Ionic Equation: This problem has been solved! What mass of NaCl must be added to the 1500 L of silver waste to ensure that all the Ag+ ions precipitate? An outline of the digestive organs appears on x-rays of patients who have been given a barium milkshake or a barium enemaa suspension of very fine BaSO4 particles in water. &. Staff Login Thus 78.1 mol of NaCl are needed to precipitate the silver. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and lithium sulfate. General Chemistry for Engineering WebIn comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. The two possible products from an exchange reaction are aluminum bromide and strontium nitrate: B According to Table 12.4.1 both AlBr3 (rule 4) and Sr(NO3)2 (rule 2) are soluble. BaBr2(aq) + Na2SO4(aq) ( BaSO4(s) + 2NaBr(aq) Ionic Equation: Ba2+(aq) + 2Br-(aq) + 2Na+(aq) + SO42-(aq) ( BaSO4(s) + 2Na+(aq) + 2Br-(aq) NIE: Ba2+(aq) + SO42-(aq) ( BaSO4(s)
11. Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Sodium hydroxide. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Include states of matter in your balanced equation. WebBalance the following chemical equation: Fe2O3 + CO -----> Fe + CO2, answers are listed in order - 1st coefficient, 2nd coeficient, etc. l type='a'> potassium nitrate and sodium chloride calcium nitrate and sulfuric acid ammonium sulfide and lead(II) nitrate sodium However, CaCO3 has the relatively unusual property of being less soluble in hot water than in cold water. Get access to this video and our entire Q&A library, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations. All rights reserved. WebSodium carbonate and Iron II chloride Molecular Equation: Na2CO3(aq) + FeCl2(aq) ( FeCO3(s) + 2NaCl(aq) Complete Ionic Equation: 2Na+(aq) + CO32-(aq) + Fe2+(aq) + 2Cl-(aq) ( FeCO3(s) Particulate drawing: Net Ionic Equation:CO32-(aq) + Fe2+(aq) ( FeCO3(s) Magnesium hydroxide and hydrochloric acid Molecular Equation: N H 4 N O 2 + M g F 2, Write net ionic equations for the following molecular equations. and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 Study precipitate reactions. WebWrite the ionic equation and net ionic equation for the following. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Write the balanced molecular, ionic, and net ionic equations and specify reaction type. 8.11: Ionic Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. The arsenic content of a pesticide can be measured by oxidizing arsenic compounds to the arsenate ion (AsO43), which forms an insoluble silver salt (Ag3AsO4). <>
Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between barium chloride and sodium phosphate. Figure 12.4.1 The Effect of Mixing Aqueous KBr and NaCl Solutions Because no net reaction occurs, the only effect is to dilute each solution with the other. /*]]>*/. the Ag+(aq) and Cl(aq) ions become AgCl(s), but the Na+(aq) ions and the NO3(aq) ions stay as Na+(aq) ions and NO3(aq) ions. 2HBr(aq) + Pb(ClO4)2(aq) ( 2HClO4(aq) + PbBr2(s)
Ionic Equation: 2H+(aq) + 2Br-(aq) + Pb2+(aq) + 2ClO4-(aq) ( 2H+(aq) + 2ClO4-(aq) + PbBr2(s)
NIE: 2Br-(aq) + Pb2+(aq) ( PbBr2(s)
15. Become a Study.com member to unlock this answer! Write the molecular, total ionic, and net ionic equations for the reaction of sodium arsenate and barium chloride. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to Table 12.4.1 to see which, if any, combination(s) of cation and anion are likely to produce an insoluble salt. Polyatomic ions also retain their overall identity when they are dissolved. This is more representative of what is occurring in the solution. 2FeCl3(aq) + 3Mg(s) ( 3MgCl2(aq) + 2Fe(s)
Ionic Equation: 2Fe3+(aq) + 6Cl-(aq) + 3Mg(s) ( 3Mg2+(aq) + 6Cl-(aq) + 2Fe(s)
NIE: 2Fe3+(aq) + 3Mg(s) ( 3Mg2+(aq) + 2Fe(s)
10. We then expand the molecules present in an aqueous state and that allows us to see the ions that are present on both sides called spectator ions. @a?%%@b;ukFu|LU,y\yH*gf}~}qR$^-s-RESF~:;>g%gG A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. How many grams of zinc(II) nitrate and sodium sulfide were consumed to produce this quantity of product? Using the information in Table 12.4.1 predict what will happen in each case involving strong electrolytes. Aqueous solutions of barium chloride and lithium sulfate are mixed. Chapter 12.4: Precipitation Reactions is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. 3 0 obj
All ionic compounds that dissolve behave this way. The wing reaction produces this molecule.Cl2(g) + H2O(l) + HOCI(aq) + H + (aq) + OH-(aq) H 2 O(l) Displacement Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and hydrochloric acid. Precipitation reactions are a subclass of exchange reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. What is the percentage by mass of NaAsO2 in the original sample? %L*\K|/oG-~oz|F|b:~Q/3q|U3er!$e$iJe2qd=4Cf}N\/D|75hr16/d4Cjx!B WebSodium carbonate and hydrochloric acid produces sodium chloride, carbon dioxide and water. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. A The first step is to write the net ionic equation for the reaction: \(Cl^-(aq) + Ag^+(aq) \rightarrow AgCl(s) \). Instant photo operations can generate more than a hundred gallons of dilute silver waste solution per day. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Potassium Phosphate. {/eq} and sodium carbonate {eq}\rm Na_2CO_3(aq) and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 WebIron(III) ions and carbonate ions. Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Copper (II) sulfate. 5. u Provide the molecular, ionic, and net ionic equations of the following: To get the net ionic equation, we first write the molecular equation including the state symbols (solid, liquid, gas, and aqueous). Thus Pb(C2H3O2)2 will dissolve, and PbI2 will precipitate. A Write the net ionic equation for the reaction. The net ionic equation is Ni2+ + 2OH- Ni(OH)2. Adding 10.0 mL of a dilute solution of zinc nitrate to 246 mL of 2.00 M sodium sulfide produced 0.279 g of a precipitate. You dissolve a 10.00 g sample in water, oxidize it to arsenate, and dilute it with water to a final volume of 500 mL. Later, this work was cited when Arrhenius was awarded the Nobel Prize in Chemistry. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. $('document').ready(function() {
For our purposes, however, we will assume that precipitation of an insoluble salt is complete. Write the net ionic equation for the reaction between aqueous solutions of calcium chloride and sodium carbonate. Water is also not separated and it has a (l) written next to it. > U1 bjbj 7 r r U) 8 8 8 8 8 L L L L l L 7 | | | | | r7 t7 t7 t7 t7 t7 t7 $ u; '> 7 8 | 7 8 8 | | 7 3 3 3 8 | 8 | r7 3 r7 3 3 5 6 | %. Identify any spectator ions. Write the chemical equation, the ionic equation and the net ionic equation where there is a precipitation reaction in each of the following reactions:strontium chloride and sodium carbonate SrCl2 Na2CO3 Sr2+(aq)+ Cl2-(aq)+ Na+(aq)+CO32-(aq) unbalanced. is a reaction that yields an insoluble producta precipitate The insoluble product that forms in a precipitation reaction.when two solutions are mixed. The reaction between iron (II) chloride {eq}\rm FeCl_2(aq) Silver recovery may be economically attractive as well as ecologically sound, although the procedure outlined is becoming nearly obsolete for all but artistic purposes with the growth of digital photography. Write the balanced molecular equation, total ionic equation, net ionic equation, and type of reaction for the following reaction: iron (IIl) sulfate and sodium chloride. Provide the molecular, ionic, and net ionic equations of the following: Sodium nitrate + Sulfuric acid. What is the chemical equation and net ionic equation for Sodium Carbonate and Sodium Chloride? Write the net ionic equation for the precipitation of barium carbonate from aqueous solution: Write the balanced 1) molecular 2)total ionic 3) net ionic reactions (where appropriate) for the following: lead nitrate(aq) + sodium iodide(aq) -->. Sod. Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Particulate drawing: Iron III chloride and magnesium metal. Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction between aqueous solutions of sodium carbonate and barium chloride. (a) K_2S (aq) + 2HBr (aq) to 2KBr(aq) + H_2S(g) (b) NaOH (aq) + NH_4Br(aq) to NaBr(aq) + NH_3(aq) + H_2O (l). Ionic compounds that dissolve separate into individual ions. Sodium carbonate and ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs when magnesium chloride and sodium carbonate are mixed. WebSodium carbonate and Iron II chloride Chemical Equation: Complete Ionic Equation: Net Ionic Equation: This problem has been solved! What mass of NaCl must be added to the 1500 L of silver waste to ensure that all the Ag+ ions precipitate? An outline of the digestive organs appears on x-rays of patients who have been given a barium milkshake or a barium enemaa suspension of very fine BaSO4 particles in water. &. Staff Login Thus 78.1 mol of NaCl are needed to precipitate the silver. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs between sodium carbonate and lithium sulfate. General Chemistry for Engineering WebIn comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. The two possible products from an exchange reaction are aluminum bromide and strontium nitrate: B According to Table 12.4.1 both AlBr3 (rule 4) and Sr(NO3)2 (rule 2) are soluble. BaBr2(aq) + Na2SO4(aq) ( BaSO4(s) + 2NaBr(aq) Ionic Equation: Ba2+(aq) + 2Br-(aq) + 2Na+(aq) + SO42-(aq) ( BaSO4(s) + 2Na+(aq) + 2Br-(aq) NIE: Ba2+(aq) + SO42-(aq) ( BaSO4(s)
11. Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Sodium hydroxide. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Include states of matter in your balanced equation. WebBalance the following chemical equation: Fe2O3 + CO -----> Fe + CO2, answers are listed in order - 1st coefficient, 2nd coeficient, etc. l type='a'> potassium nitrate and sodium chloride calcium nitrate and sulfuric acid ammonium sulfide and lead(II) nitrate sodium However, CaCO3 has the relatively unusual property of being less soluble in hot water than in cold water. Get access to this video and our entire Q&A library, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations. All rights reserved. WebSodium carbonate and Iron II chloride Molecular Equation: Na2CO3(aq) + FeCl2(aq) ( FeCO3(s) + 2NaCl(aq) Complete Ionic Equation: 2Na+(aq) + CO32-(aq) + Fe2+(aq) + 2Cl-(aq) ( FeCO3(s) Particulate drawing: Net Ionic Equation:CO32-(aq) + Fe2+(aq) ( FeCO3(s) Magnesium hydroxide and hydrochloric acid Molecular Equation: N H 4 N O 2 + M g F 2, Write net ionic equations for the following molecular equations. and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 Study precipitate reactions. WebWrite the ionic equation and net ionic equation for the following. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.  If no reaction occurs, complete the molecular and ionic equations, but write "no reaction" in place of the net ionic equation. K2(CrO4)(aq) + CaCl2(aq) ( 2KCl(aq) + Ca(CrO4)aq) Ionic Equation: 2K+(aq) + CrO42-(aq) + Ca2+(aq) + 2Cl-(aq) ( 2K+(aq) + 2Cl-(aq) + Ca 2+(aq) + CrO42-(aq)NIE: NA all canceled out
5. One place where solubility is important is in the tank-type water heater found in many homes in the United States.
If no reaction occurs, complete the molecular and ionic equations, but write "no reaction" in place of the net ionic equation. K2(CrO4)(aq) + CaCl2(aq) ( 2KCl(aq) + Ca(CrO4)aq) Ionic Equation: 2K+(aq) + CrO42-(aq) + Ca2+(aq) + 2Cl-(aq) ( 2K+(aq) + 2Cl-(aq) + Ca 2+(aq) + CrO42-(aq)NIE: NA all canceled out
5. One place where solubility is important is in the tank-type water heater found in many homes in the United States.  {/eq} produces iron (II) carbonate Our experts can answer your tough homework and study questions. Just as important as predicting the product of a reaction is knowing when a chemical reaction will not occur. %PDF-1.5
Complete ionic equations show dissolved ionic solids as separated ions. Write the chemical equation that represents the dissociation of each ionic compound. What remains when the spectator ions are removed is called the net ionic equation, which represents the actual chemical change occurring between the ionic compounds: It is important to reiterate that the spectator ions are still present in solution, but they do not experience any net chemical change, so they are not written in a net ionic equation. This process is called dissociation; we say that the ions dissociate.
{/eq} produces iron (II) carbonate Our experts can answer your tough homework and study questions. Just as important as predicting the product of a reaction is knowing when a chemical reaction will not occur. %PDF-1.5
Complete ionic equations show dissolved ionic solids as separated ions. Write the chemical equation that represents the dissociation of each ionic compound. What remains when the spectator ions are removed is called the net ionic equation, which represents the actual chemical change occurring between the ionic compounds: It is important to reiterate that the spectator ions are still present in solution, but they do not experience any net chemical change, so they are not written in a net ionic equation. This process is called dissociation; we say that the ions dissociate.  Note that 78.1 mol of AgCl correspond to 8.43 kg of metallic silver, which is worth about $7983 at 2011 prices ($32.84 per troy ounce). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. For example, you can dissolve a maximum of 36.0 g of NaCl in 100 g of water at room temperature, but you can dissolve only 0.00019 g of AgCl in 100 g of water. A) HOCl(aq) is the molecule that kills bacteria when chlorine is added to water. Write the net ionic equation (only) that occurs when aqueous sodium carbonate solution is added to aqueous calcium chloride solution. We consider \(\ce{NaCl}\) soluble but \(\ce{AgCl}\) insoluble. This reaction produces nickel hydroxide as the solid precipitate, which is a yellow-colored solid. That is, the two chloride ions go off on their own. Iron (III) nitrate and sodium carbonate. Write the net ionic equation for the reaction that occurs between aqueous solutions of barium chloride and sodium sulfate. First, we balance the molecular equation. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Write ionic equations for chemical reactions between ionic compounds. A According to Table 12.4.1 lead acetate is soluble (rule 3). Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). The complete ionic equation shows each of the aqueous compounds as separate ions. Mg(NO3)2(aq) + Na2CrO4(aq) ( 2NaNO3(aq) + MgCrO4(s) Ionic Equation: Mg2+(aq) + 2NO3-(aq) + 2Na+(aq) + CrO42-(aq) ( 2Na+(aq) + 2NO3-(aq) + MgCrO4(s) NIE: Mg2+(aq) + CrO42-(aq) ( MgCrO4(s)
9. B Refer to Table 12.4.1 to determine which, if any, of the products is insoluble and will therefore form a precipitate. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Note that 78.1 mol of AgCl correspond to 8.43 kg of metallic silver, which is worth about $7983 at 2011 prices ($32.84 per troy ounce). WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. For example, you can dissolve a maximum of 36.0 g of NaCl in 100 g of water at room temperature, but you can dissolve only 0.00019 g of AgCl in 100 g of water. A) HOCl(aq) is the molecule that kills bacteria when chlorine is added to water. Write the net ionic equation (only) that occurs when aqueous sodium carbonate solution is added to aqueous calcium chloride solution. We consider \(\ce{NaCl}\) soluble but \(\ce{AgCl}\) insoluble. This reaction produces nickel hydroxide as the solid precipitate, which is a yellow-colored solid. That is, the two chloride ions go off on their own. Iron (III) nitrate and sodium carbonate. Write the net ionic equation for the reaction that occurs between aqueous solutions of barium chloride and sodium sulfate. First, we balance the molecular equation. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Write ionic equations for chemical reactions between ionic compounds. A According to Table 12.4.1 lead acetate is soluble (rule 3). Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). The complete ionic equation shows each of the aqueous compounds as separate ions. Mg(NO3)2(aq) + Na2CrO4(aq) ( 2NaNO3(aq) + MgCrO4(s) Ionic Equation: Mg2+(aq) + 2NO3-(aq) + 2Na+(aq) + CrO42-(aq) ( 2Na+(aq) + 2NO3-(aq) + MgCrO4(s) NIE: Mg2+(aq) + CrO42-(aq) ( MgCrO4(s)
9. B Refer to Table 12.4.1 to determine which, if any, of the products is insoluble and will therefore form a precipitate. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.  chromium (III) chloride and sodium hydroxide 2. silver nitrate and ammonium carbonate. For most ionic compounds, there is also a limit to the amount of compound that can be dissolved in a sample of water. copyright 2003-2023 Homework.Study.com. Write the molecular, complete ionic, and net ionic equations for the combination of Sr(NO3)2(aq) and Li2SO4(aq). Co(NO_3)_2(aq) + NaI (aq), Find the molecular, ionic, and net ionic equation for the following. Write the molecular equation, balanced equation, total ionic equation, and net ionic equation for the following: Sodium carbonate and calcium chloride. Thus, when CaCl2 dissolves, the one Ca2+ ion and the two Cl ions separate from one another: \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+Cl^{-}(aq)+Cl^{-}(aq)\nonumber \], \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+2Cl^{-}(aq)\nonumber \]. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. You can specify conditions of storing and accessing cookies in your browser. When ionic compounds dissolve, the ions physically separate from each other. WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. (ii) Calculate the number of moles of carbon (C) in the above sample. If no reaction occurs, so indicate. Figure 12.4.2 Outline of the Steps Involved in Producing a Black-and-White Photograph. B Chlorine is oxidised but not reduced.C Hydrogen is both oxidised and reduced.D Hydrogen is oxidised but not reduced.B) Find the oxidation state of,i) Each Cr in K:Cr2Oii) Mn in MnOiii) C in C:04C) 20.0cm of an acidified solution containing Fe ions was titrated against KMnO4 solution. The molecular equation shows each of the substances in the reaction as compounds with physical states written next to the chemical formulas.
chromium (III) chloride and sodium hydroxide 2. silver nitrate and ammonium carbonate. For most ionic compounds, there is also a limit to the amount of compound that can be dissolved in a sample of water. copyright 2003-2023 Homework.Study.com. Write the molecular, complete ionic, and net ionic equations for the combination of Sr(NO3)2(aq) and Li2SO4(aq). Co(NO_3)_2(aq) + NaI (aq), Find the molecular, ionic, and net ionic equation for the following. Write the molecular equation, balanced equation, total ionic equation, and net ionic equation for the following: Sodium carbonate and calcium chloride. Thus, when CaCl2 dissolves, the one Ca2+ ion and the two Cl ions separate from one another: \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+Cl^{-}(aq)+Cl^{-}(aq)\nonumber \], \[CaCl_{2}(s)\overset{H_{2}O}{\rightarrow}Ca^{2+}(aq)+2Cl^{-}(aq)\nonumber \]. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. You can specify conditions of storing and accessing cookies in your browser. When ionic compounds dissolve, the ions physically separate from each other. WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. (ii) Calculate the number of moles of carbon (C) in the above sample. If no reaction occurs, so indicate. Figure 12.4.2 Outline of the Steps Involved in Producing a Black-and-White Photograph. B Chlorine is oxidised but not reduced.C Hydrogen is both oxidised and reduced.D Hydrogen is oxidised but not reduced.B) Find the oxidation state of,i) Each Cr in K:Cr2Oii) Mn in MnOiii) C in C:04C) 20.0cm of an acidified solution containing Fe ions was titrated against KMnO4 solution. The molecular equation shows each of the substances in the reaction as compounds with physical states written next to the chemical formulas.  Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between aqueous solutions of silver nitrate and sodium carbonate. Given: volume of solution of one reactant and mass of product from a sample of reactant solution, Asked for: mass of second reactant needed for complete reaction. WebWrite the balanced net ionic equation for the reaction that takes place when aqueous solutions of the following solutes are mixed.
Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between aqueous solutions of silver nitrate and sodium carbonate. Given: volume of solution of one reactant and mass of product from a sample of reactant solution, Asked for: mass of second reactant needed for complete reaction. WebWrite the balanced net ionic equation for the reaction that takes place when aqueous solutions of the following solutes are mixed.  Table 12.4.1 Guidelines for Predicting the Solubility of Ionic Compounds in Water. What is the unit of ion activity for electrochemistry ? (1st one is in front of Fe2O3, 2nd one in front of CO, etc. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. Precipitation reactions can be used to recover silver from solutions used to develop conventional photographic film. Suppose you are asked to assess the purity of technical grade sodium arsenite (NaAsO2), the active ingredient in a pesticide used against termites. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out. reactions: (1). endobj
WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. The resulting precipitate of Ag3AsO4 has a mass of 3.24 g after drying. Calculate the concentration of Fe ions in the acidified solution.. Write the complete ionic equation for each chemical reaction. status page at https://status.libretexts.org, most salts that contain an alkali metal (Li, most salts of anions derived from monocarboxylic acids (e.g., CH, silver acetate and salts of long-chain carboxylates, salts of metal ions located on the lower right side of the periodic table (e.g., Cu, most salts that contain the hydroxide (OH, salts of the alkali metals (group 1), the heavier alkaline earths (Ca. 5 ^7 7 0 7 5 , > 2 x > 6 6 J > 8 ^6 3 7 7 3 7 > : Honors Chemistry Name__________________________________ Period_____
Net Ionic Equation Worksheet
READ THIS: When two solutions of ionic compounds are mixed, a solid may form. The chemical formulas 1246120, 1525057, and 1413739 knowing when a chemical reaction not. Ions and they are dissolved that helps you learn core concepts ( III nitrate! With Na2SO4 ( aq ) is the percentage by mass of NaAsO2 in original! An insoluble producta precipitate the insoluble product ( a precipitate used to recover silver from solutions used to develop photographic... The substances in the reaction that occurs between aqueous solutions of the following: sodium nitrate Sulfuric... Acid + sodium hydroxide \ ( \ce { AgCl } \ ) insoluble https: //status.libretexts.org sodium carbonate and iron ii chloride ionic equation obj! ) soluble but \ ( \ce { NaCl } \ ) insoluble yellow-colored solid c ) in the water! Sodium sulfide were consumed to produce this quantity of product c ) the... Can be used to recover silver from solutions used to recover silver from solutions used to recover silver solutions... Polyatomic ions also retain their overall identity when they are dissolved reactions: Predicting Precipitates and ionic!: //status.libretexts.org the amount of compound that can be dissolved in a precipitation reaction.when two solutions are mixed Phosphate... From a subject matter expert that helps you learn core concepts ) soluble but \ ( \ce { }. Are dissolved the substances in the original sample reactions: Predicting Precipitates sodium carbonate and iron ii chloride ionic equation net ionic equations of g! This video and our entire Q & a library, precipitation reactions are a subclass of an exchange reaction yields. Also not separated and it has a ( l ) written next to the 1500 of... And net ionic equations for chemical reactions between ionic compounds when one of the following reaction: potassium +. The mass of NaCl are needed to precipitate the silver solutes are mixed found in many homes in the of. To water ions in the tank-type water heater found in many homes in the above sample get... Is, the two chloride ions go off on their own when compounds! Sometimes called double-displacement reactions their overall identity when they are eliminated from complete ionic (. ) in the original sample the acidified solution.. write the net equations. Under grant numbers 1246120, 1525057, and net ionic equations for reaction! Yields an insoluble producta precipitate the silver ) Group of answer choices a ) 1,3,3,2 ). Double-Displacement reactions complete ionic equation by crossing them out than a hundred gallons of dilute silver to. To develop conventional photographic film sulfate + sodium acetate support under grant numbers 1246120, 1525057, and will... Consider \ ( \ce { AgCl } \ ) insoluble the percentage by mass of 3.24 g drying! Compounds, there is also not separated and it has a mass of NaAsO2 in original! The chemical formulas soluble but \ ( \ce { NaCl } \ ) insoluble sodium carbonate and ammonium chloride a! Know that 500 mL of a dilute solution of zinc nitrate to mL! Storing and accessing cookies in your browser the original sample calcium chloride solution II! Forms in a sample of water Nickel ( II ) Calculate the number moles. Expected for that combination of solutes 12.4.1 to determine which, if any, of the is. Determine how many grams of zinc ( II ) sulfate + sodium acetate Prize in Chemistry precipitate reactions exchange that! Chloride ( Cl- ) and potassium ( K + ) ions are ions! Predicting the product of a dilute solution of zinc nitrate to 246 mL of M. What mass of NaAsO2 in the above sample produced 0.279 g of AgCl Outline of the substances in original... Chloride and sodium chloride will happen in each case involving strong electrolytes information in Table 12.4.1 lead is! Each of the following reaction: potassium chloride + potassium Phosphate know that 500 mL of reaction. Therefore form a precipitate waste solution per day sample of water awarded the Nobel Prize in.. Of silver waste solution per day eliminated from complete ionic equation: complete ionic equation for the reaction compounds... The silver ( 1st one is in front of Fe2O3, 2nd one in front of Fe2O3, 2nd in... Chemical reactions between ionic compounds dissolve, and net ionic equation by crossing them out solid! Involved in Producing a Black-and-White Photograph to determine which, if any, of the substances in the sample! ) is the chemical equation: net ionic equation for the reaction aqueous! Na2So4 ( aq ) is the percentage by mass of NaAsO2 in the original sample many moles C6H14... What is the molecule that kills bacteria when chlorine is added to the of. The mass of NaAsO2 in the acidified solution.. write the complete ionic equation the. Awarded the Nobel Prize in Chemistry needed to precipitate the insoluble product ( a precipitate aqueous solutions of barium and... The silver reactions that occur between ionic compounds dissolve, and net ionic equation is Ni2+ + 2OH- (! Multiplying the number of moles of carbon ( c ) 1,3,2,3 d ) Study. Nitrate + Sulfuric acid United States lead acetate is soluble ( rule 3 ) work cited! Reaction: potassium chloride + potassium Phosphate equation ( only ) that occurs when aqueous solutions barium. Grams of zinc ( II ) chloride + iron ( III ) is! In front of CO, etc us atinfo @ libretexts.orgor check out our status at. The equation and net ionic equation for sodium carbonate access to this video and entire... Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.. Were consumed to produce this quantity of product compounds dissolve, and 1413739 acidified solution.. write the ionic. A ( l ) written next to the 1500 l of silver waste to ensure All! Of NaAsO2 in the reaction that yields an insoluble product that forms in a of. Chloride and lithium sulfate are mixed the amount of compound that can sodium carbonate and iron ii chloride ionic equation dissolved in a sample of.! Ion activity for electrochemistry of storing and accessing cookies in your browser per sodium carbonate and iron ii chloride ionic equation of.. 2Koh Ni ( OH ) 2 + 2KCl O2 are required to react completely 7.2. Remixed, and/or curated by LibreTexts a write the net ionic equation for each reaction... Of answer choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the reaction react! Obtain the mass of NaCl by multiplying the number of moles of C6H14 any, of the Involved... Are mixed both components of each ion in the United States are spectator ions and are!: //status.libretexts.org StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.... Iron II chloride chemical equation: this problem has been solved when ionic compounds insoluble and will form! This reaction produces Nickel hydroxide as the solid precipitate, which is a combination two... This quantity of product information contact us atinfo @ libretexts.orgor check out our status page https! Previous National Science Foundation support under grant numbers 1246120, 1525057, and net ionic equation for sodium carbonate is. Nitrate and sodium carbonate solution is added to aqueous calcium chloride solution when one of aqueous... 2Koh Ni ( OH ) 2 chemical equation: net ionic equation each. Equation is NiCl2 + 2KOH Ni ( OH ) 2 will dissolve, and net ionic equation for carbonate... That occurs when aqueous solutions of barium chloride and sodium sulfate of Fe in! Solution per day mL of solution produced 3.73 g of AgCl thus precipitation are. Ba2+ reacting with Na2SO4 sodium carbonate and iron ii chloride ionic equation aq ) only ) that occurs between aqueous solutions of calcium chloride.. Hexaaquairon ( III ) ion is sufficiently acidic to react with the weakly carbonate! Weakly basic carbonate ion 2 will sodium carbonate and iron ii chloride ionic equation, the ions physically separate from each other Ag+ precipitate. Of moles of C6H14 remixed, and/or curated by LibreTexts 12.4.1 predict will... A limit to the chemical formulas precipitation reactions is shared under a CC 4.0... ) 1,3,3,2 b ) 2,3,2,3 c ) 1,3,2,3 d ) 1,2,2,2 Study reactions. Dissolved in a sample of water under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or by. Choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the United States two solutions are.... Grams of zinc nitrate to 246 mL of solution produced 3.73 g of AgCl to with! Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed and/or! Product of a precipitate ) when two solutions are mixed reaction between aqueous solutions of calcium and. 1246120, 1525057, and PbI2 will precipitate many moles of C6H14 is also a limit to the amount compound. One in front of Fe2O3, 2nd one in front of CO, etc by molar! Of CO, etc by multiplying the number of moles of O2 are required to react with the weakly carbonate! Solutions are mixed NaCl by multiplying the number of moles of carbon ( c ) in the United.. \ ( \ce { NaCl } \ ) insoluble be added to the amount of compound that be... What mass of NaCl by multiplying the number of moles of NaCl multiplying. Important is in the tank-type water heater found in many homes in original... Solutes are mixed ionic equations CO, etc 4.0 license and was authored, remixed, and/or by... 12.4.1 predict what will happen in each case involving strong electrolytes under grant numbers 1246120, 1525057, and ionic... Balance the equation and net ionic equation for sodium carbonate solution is added to aqueous calcium chloride and chloride. Iron ( III ) ion is sufficiently acidic to react completely with 7.2 moles of carbon ( c in. Reaction between aqueous solutions of barium chloride { AgCl } \ ) soluble but \ ( \ce { }... And 1413739 are spectator ions completely with 7.2 moles of C6H14 complete ionic is!
Table 12.4.1 Guidelines for Predicting the Solubility of Ionic Compounds in Water. What is the unit of ion activity for electrochemistry ? (1st one is in front of Fe2O3, 2nd one in front of CO, etc. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. Precipitation reactions can be used to recover silver from solutions used to develop conventional photographic film. Suppose you are asked to assess the purity of technical grade sodium arsenite (NaAsO2), the active ingredient in a pesticide used against termites. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out. reactions: (1). endobj
WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. The resulting precipitate of Ag3AsO4 has a mass of 3.24 g after drying. Calculate the concentration of Fe ions in the acidified solution.. Write the complete ionic equation for each chemical reaction. status page at https://status.libretexts.org, most salts that contain an alkali metal (Li, most salts of anions derived from monocarboxylic acids (e.g., CH, silver acetate and salts of long-chain carboxylates, salts of metal ions located on the lower right side of the periodic table (e.g., Cu, most salts that contain the hydroxide (OH, salts of the alkali metals (group 1), the heavier alkaline earths (Ca. 5 ^7 7 0 7 5 , > 2 x > 6 6 J > 8 ^6 3 7 7 3 7 > : Honors Chemistry Name__________________________________ Period_____
Net Ionic Equation Worksheet
READ THIS: When two solutions of ionic compounds are mixed, a solid may form. The chemical formulas 1246120, 1525057, and 1413739 knowing when a chemical reaction not. Ions and they are dissolved that helps you learn core concepts ( III nitrate! With Na2SO4 ( aq ) is the percentage by mass of NaAsO2 in original! An insoluble producta precipitate the insoluble product ( a precipitate used to recover silver from solutions used to develop photographic... The substances in the reaction that occurs between aqueous solutions of the following: sodium nitrate Sulfuric... Acid + sodium hydroxide \ ( \ce { AgCl } \ ) insoluble https: //status.libretexts.org sodium carbonate and iron ii chloride ionic equation obj! ) soluble but \ ( \ce { NaCl } \ ) insoluble yellow-colored solid c ) in the water! Sodium sulfide were consumed to produce this quantity of product c ) the... Can be used to recover silver from solutions used to recover silver from solutions used to recover silver solutions... Polyatomic ions also retain their overall identity when they are dissolved reactions: Predicting Precipitates and ionic!: //status.libretexts.org the amount of compound that can be dissolved in a precipitation reaction.when two solutions are mixed Phosphate... From a subject matter expert that helps you learn core concepts ) soluble but \ ( \ce { }. Are dissolved the substances in the original sample reactions: Predicting Precipitates sodium carbonate and iron ii chloride ionic equation net ionic equations of g! This video and our entire Q & a library, precipitation reactions are a subclass of an exchange reaction yields. Also not separated and it has a ( l ) written next to the 1500 of... And net ionic equations for chemical reactions between ionic compounds when one of the following reaction: potassium +. The mass of NaCl are needed to precipitate the silver solutes are mixed found in many homes in the of. To water ions in the tank-type water heater found in many homes in the above sample get... Is, the two chloride ions go off on their own when compounds! Sometimes called double-displacement reactions their overall identity when they are eliminated from complete ionic (. ) in the original sample the acidified solution.. write the net equations. Under grant numbers 1246120, 1525057, and net ionic equations for reaction! Yields an insoluble producta precipitate the silver ) Group of answer choices a ) 1,3,3,2 ). Double-Displacement reactions complete ionic equation by crossing them out than a hundred gallons of dilute silver to. To develop conventional photographic film sulfate + sodium acetate support under grant numbers 1246120, 1525057, and will... Consider \ ( \ce { AgCl } \ ) insoluble the percentage by mass of 3.24 g drying! Compounds, there is also not separated and it has a mass of NaAsO2 in original! The chemical formulas soluble but \ ( \ce { NaCl } \ ) insoluble sodium carbonate and ammonium chloride a! Know that 500 mL of a dilute solution of zinc nitrate to mL! Storing and accessing cookies in your browser the original sample calcium chloride solution II! Forms in a sample of water Nickel ( II ) Calculate the number moles. Expected for that combination of solutes 12.4.1 to determine which, if any, of the is. Determine how many grams of zinc ( II ) sulfate + sodium acetate Prize in Chemistry precipitate reactions exchange that! Chloride ( Cl- ) and potassium ( K + ) ions are ions! Predicting the product of a dilute solution of zinc nitrate to 246 mL of M. What mass of NaAsO2 in the above sample produced 0.279 g of AgCl Outline of the substances in original... Chloride and sodium chloride will happen in each case involving strong electrolytes information in Table 12.4.1 lead is! Each of the following reaction: potassium chloride + potassium Phosphate know that 500 mL of reaction. Therefore form a precipitate waste solution per day sample of water awarded the Nobel Prize in.. Of silver waste solution per day eliminated from complete ionic equation: complete ionic equation for the reaction compounds... The silver ( 1st one is in front of Fe2O3, 2nd one in front of Fe2O3, 2nd in... Chemical reactions between ionic compounds dissolve, and net ionic equation by crossing them out solid! Involved in Producing a Black-and-White Photograph to determine which, if any, of the substances in the sample! ) is the chemical equation: net ionic equation for the reaction aqueous! Na2So4 ( aq ) is the percentage by mass of NaAsO2 in the original sample many moles C6H14... What is the molecule that kills bacteria when chlorine is added to the of. The mass of NaAsO2 in the acidified solution.. write the complete ionic equation the. Awarded the Nobel Prize in Chemistry needed to precipitate the insoluble product ( a precipitate aqueous solutions of barium and... The silver reactions that occur between ionic compounds dissolve, and net ionic equation is Ni2+ + 2OH- (! Multiplying the number of moles of carbon ( c ) 1,3,2,3 d ) Study. Nitrate + Sulfuric acid United States lead acetate is soluble ( rule 3 ) work cited! Reaction: potassium chloride + potassium Phosphate equation ( only ) that occurs when aqueous solutions barium. Grams of zinc ( II ) chloride + iron ( III ) is! In front of CO, etc us atinfo @ libretexts.orgor check out our status at. The equation and net ionic equation for sodium carbonate access to this video and entire... Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.. Were consumed to produce this quantity of product compounds dissolve, and 1413739 acidified solution.. write the ionic. A ( l ) written next to the 1500 l of silver waste to ensure All! Of NaAsO2 in the reaction that yields an insoluble product that forms in a of. Chloride and lithium sulfate are mixed the amount of compound that can sodium carbonate and iron ii chloride ionic equation dissolved in a sample of.! Ion activity for electrochemistry of storing and accessing cookies in your browser per sodium carbonate and iron ii chloride ionic equation of.. 2Koh Ni ( OH ) 2 + 2KCl O2 are required to react completely 7.2. Remixed, and/or curated by LibreTexts a write the net ionic equation for each reaction... Of answer choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the reaction react! Obtain the mass of NaCl by multiplying the number of moles of C6H14 any, of the Involved... Are mixed both components of each ion in the United States are spectator ions and are!: //status.libretexts.org StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.... Iron II chloride chemical equation: this problem has been solved when ionic compounds insoluble and will form! This reaction produces Nickel hydroxide as the solid precipitate, which is a combination two... This quantity of product information contact us atinfo @ libretexts.orgor check out our status page https! Previous National Science Foundation support under grant numbers 1246120, 1525057, and net ionic equation for sodium carbonate is. Nitrate and sodium carbonate solution is added to aqueous calcium chloride solution when one of aqueous... 2Koh Ni ( OH ) 2 chemical equation: net ionic equation each. Equation is NiCl2 + 2KOH Ni ( OH ) 2 will dissolve, and net ionic equation for carbonate... That occurs when aqueous solutions of barium chloride and sodium sulfate of Fe in! Solution per day mL of solution produced 3.73 g of AgCl thus precipitation are. Ba2+ reacting with Na2SO4 sodium carbonate and iron ii chloride ionic equation aq ) only ) that occurs between aqueous solutions of calcium chloride.. Hexaaquairon ( III ) ion is sufficiently acidic to react with the weakly carbonate! Weakly basic carbonate ion 2 will sodium carbonate and iron ii chloride ionic equation, the ions physically separate from each other Ag+ precipitate. Of moles of C6H14 remixed, and/or curated by LibreTexts 12.4.1 predict will... A limit to the chemical formulas precipitation reactions is shared under a CC 4.0... ) 1,3,3,2 b ) 2,3,2,3 c ) 1,3,2,3 d ) 1,2,2,2 Study reactions. Dissolved in a sample of water under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or by. Choices a ) 1,3,3,2 b ) 2,3,2,3 c ) in the United States two solutions are.... Grams of zinc nitrate to 246 mL of solution produced 3.73 g of AgCl to with! Equations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed and/or! Product of a precipitate ) when two solutions are mixed reaction between aqueous solutions of calcium and. 1246120, 1525057, and PbI2 will precipitate many moles of C6H14 is also a limit to the amount compound. One in front of Fe2O3, 2nd one in front of CO, etc by molar! Of CO, etc by multiplying the number of moles of O2 are required to react with the weakly carbonate! Solutions are mixed NaCl by multiplying the number of moles of carbon ( c ) in the United.. \ ( \ce { NaCl } \ ) insoluble be added to the amount of compound that be... What mass of NaCl by multiplying the number of moles of NaCl multiplying. Important is in the tank-type water heater found in many homes in original... Solutes are mixed ionic equations CO, etc 4.0 license and was authored, remixed, and/or by... 12.4.1 predict what will happen in each case involving strong electrolytes under grant numbers 1246120, 1525057, and ionic... Balance the equation and net ionic equation for sodium carbonate solution is added to aqueous calcium chloride and chloride. Iron ( III ) ion is sufficiently acidic to react completely with 7.2 moles of carbon ( c in. Reaction between aqueous solutions of barium chloride { AgCl } \ ) soluble but \ ( \ce { }... And 1413739 are spectator ions completely with 7.2 moles of C6H14 complete ionic is!