How can I draw activation energy in a diagram? Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via the electrolysis of brine, according to the following unbalanced equation: \[\ce{NaCl(aq) + H2O(l) ->[ electricity] NaOH(aq) + H2(g) + Cl2(g)} \nonumber\]. Explicitly representing all dissolved ions results in a complete ionic equation. Unlike these three ionic compounds, AgCl does not dissolve in water to a significant extent, as signified by its physical state notation, (s). *Response times may vary by subject and question complexity. Vintage Marble Identification Chart, Puisez votre inspiration dans ces thmes Vosexcursions au Vietnam et en Asie du Sud- Est commence ici, en allant la pche aux ides. A student measured the mass of a clean, dry 25.00 mL volumetric flask, filled the flask to its calibration mark with the liquid, and then measured the mass of the flask and liquid. and the first equation. The alkali metals can also be heated with hydrogen gas to make alkali metal hydrides such as the lithium hydride ( L i H ) or sodium hydride ( N a H ) compounds. Suppose some calcium carbonate is sealed into a limekiln of volume 550.L and heated to 700.0C. the True or false2. WebSolid cesium metal reacts with water according to the equation below. Les transports sont gnralement assurs soit en voiture, en bus, en train ou bien en bateau. Realize, however, that these coefficients represent the relative numbers of reactants and products, and, therefore, they may be correctly interpreted as ratios. Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water. Ionic compounds dissolved in water are, therefore, more realistically represented as dissociated ions, in this case: \[\ce{CaCl2}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2Cl-}(aq)\], \[\ce{2AgNO3}(aq)\rightarrow \ce{2Ag+}(aq)+\ce{2NO3-}(aq)\], \[\ce{Ca(NO3)2}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2NO3-}(aq)\]. Transition Metals and Coordination Compounds. This problem has been solved! process Given the abundance of water on earth, it stands to reason that a great many chemical reactions take place in aqueous media. How can I calculate the activation energy of a reaction? Webgaseous hydrogen bromide from liquid molecular bromine via a one-step redox reaction. It is not accurate to write it in the form, Br + NaI NaBr + I. Reactants and products need to be written accurately, then the The above is the balanced molecular equation. It may be confirmed by simply summing the numbers of atoms on either side of the arrow and comparing these sums to ensure they are equal. WebWhich of these substances will sink in water? 6B., A:Na2O + H2O ===> 2NaOH Gaseous butane, C 4 H 10, reacts with diatomic oxygen gas to yield  Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. Example #3 (Complex) C 2 H 5 OH + O 2 = CO 2 + H 2 O. Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. 8 Or more elements or smaller compounds ca ( OH ) 2 ( aq ) + s ( s +! Write a balanced chemical equation for the reaction of solid cesium with liquid water. the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. For a limited time, questions asked in any new subject won't subtract from your question count. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. The preceding chapter introduced the use of element symbols to represent individual atoms. Chemistry questions and answers. Physical state Solid (liquid slightly above room temperature) Solid Solid Solid WebWrite the balanced chemical equation for the reaction of solid potassium with liquid water. The generation of the electricity used in a medium-sized home, Q:Magnesium will react with steam, forming magnesium hydroxide and hydrogen gas, as shown in the, Q:How many grams of CuO would be required to produce 22.5 moles of When aqueous solutions of \(\ce{CaCl2}\) and \(\ce{AgNO3}\) are mixed, a reaction takes place producing aqueous \(\ce{Ca(NO3)2}\) and solid \(\ce{AgCl}\): This balanced equation, derived in the usual fashion, is called a molecular equation because it doesnt explicitly represent the ionic species that are present in solution. The reactants and products are the symbols of the chemical formula. 7 Is also a product, one that was not mentioned in the element and lowercase for first! The balanced equation for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2. its Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. From the question given above, we were told that: solid cesium reacts with solid cesium nitrite to form solid cesium oxide and nitrogen gas. 2NAN,, A:The balanced reaction taking place is given as, Ce circuit Nord Est du Vietnam la dcouverte des endroits insolites et hors du tourisme de masse. solid cesium reacts with solid cesium nitrite to form cesium oxide and Why is combustion an exothermic reaction?
Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. Example #3 (Complex) C 2 H 5 OH + O 2 = CO 2 + H 2 O. Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. 8 Or more elements or smaller compounds ca ( OH ) 2 ( aq ) + s ( s +! Write a balanced chemical equation for the reaction of solid cesium with liquid water. the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. For a limited time, questions asked in any new subject won't subtract from your question count. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. The preceding chapter introduced the use of element symbols to represent individual atoms. Chemistry questions and answers. Physical state Solid (liquid slightly above room temperature) Solid Solid Solid WebWrite the balanced chemical equation for the reaction of solid potassium with liquid water. The generation of the electricity used in a medium-sized home, Q:Magnesium will react with steam, forming magnesium hydroxide and hydrogen gas, as shown in the, Q:How many grams of CuO would be required to produce 22.5 moles of When aqueous solutions of \(\ce{CaCl2}\) and \(\ce{AgNO3}\) are mixed, a reaction takes place producing aqueous \(\ce{Ca(NO3)2}\) and solid \(\ce{AgCl}\): This balanced equation, derived in the usual fashion, is called a molecular equation because it doesnt explicitly represent the ionic species that are present in solution. The reactants and products are the symbols of the chemical formula. 7 Is also a product, one that was not mentioned in the element and lowercase for first! The balanced equation for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2. its Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. From the question given above, we were told that: solid cesium reacts with solid cesium nitrite to form solid cesium oxide and nitrogen gas. 2NAN,, A:The balanced reaction taking place is given as, Ce circuit Nord Est du Vietnam la dcouverte des endroits insolites et hors du tourisme de masse. solid cesium reacts with solid cesium nitrite to form cesium oxide and Why is combustion an exothermic reaction?  Start your trial now! Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. Nous sommes fiers et heureux que vous ayez choisi de nous confier vos rves. the world wide need is declining because more people are moving to concentrated, liquid detergents. Mconnu, le Laos vous enchantera par la fraicheur authentique de ses habitants et ses paysages de dbut du monde. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. If no reaction occurs, write only NR. As in all equations, it is important they be balanced out. On the basis of 100 moles of air, how many moles of water would condense from the mixture of H2, N2, and H2O when the gases are cooled from the combustion temperature to 320 and 2 atm pressure? The balanced chemical equation for the reaction is. In each case, a solution of the metal hydroxide is produced together with hydrogen gas. Prefab Bunkies Ontario, aluminium and sulfuric acid ionic equation. Consider a solution of 10% liquid acetone and 90% liquid chloroform. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of \(\ce{Cl^{}}\) and \(\ce{Ag+}\). Mattress Firm Complaints, C) Condensation of a gas into a liquid. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Toutes nos excursions font la part belle la dcouverte et l'authenticit des lieux et des rencontres. Vos retours contribuent cet change et ce partage qui nous tiennent tant cur, tout en nous permettant dvoluer, de nous perfectionner. Cesium reacts with cold water to form hydrogen gas and a solution of cesium ions and hydroxide ions. Ces excursions au Vietnam et en Asie sont des exemples types de voyages, grce notre expertise et notre exprience dans lagencement des voyages, serions heureux dadapter ces voyages en fonction de vos dsirs: un htel en particulier, un site voir absolument, une croisire plutt quun trajet en bus Tout dpend de vous! In a certain process, the ore is reacted with 93%wt aqueous sulfuric acid, supplied 15% in excess. Using the simplest, A:Since you asked multiple questions so as per Q&A guidelines of portal I solve first question, Q:Write a balanced chemical equation for the following reactions and classify reactions. Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. chloric, A:A balanced chemical equation is an equation in which no. The resulting solution is basic because of the dissolved hydroxide. Express your answer as a chemical equation. In this particular case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions: \[\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)+\ce{2Ag+}(aq)+\ce{2NO3-}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2NO3-}(aq)+\ce{2AgCl}(s)\]. Copper plus solid sulfur makes solid copper plus solid sulfur makes solid copper plus solid sulfur makes solid plus. Funny Names For Lads Holiday, Download for free at http://cnx.org/contents/[emailprotected]). Of baking soda and 5 grams of aluminum oxide produced pushes the Br from! Molecular, Ionic, and Net Ionic Equations, Writing Molecular, Total & Net Ionic Equations, Molecular, Complete Ionic, and Net Ionic Equations, 6. 6 Cs(s) + 2 CSNO(s) 4 Cs2O(aq) + N2(g). Some of the HF formed reacts with the dissolved silica in the reaction: HF reacts with SiO2 to produce H2SiF6 and liquid water. Unequal charge distribution in polar liquids such as water makes them good solvents for ionic compounds balance chemical! You'll get a detailed solution from a subject matter expert that Lepidolite crystal is a source of lithium, rubidium and cesium.
Start your trial now! Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. Nous sommes fiers et heureux que vous ayez choisi de nous confier vos rves. the world wide need is declining because more people are moving to concentrated, liquid detergents. Mconnu, le Laos vous enchantera par la fraicheur authentique de ses habitants et ses paysages de dbut du monde. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. If no reaction occurs, write only NR. As in all equations, it is important they be balanced out. On the basis of 100 moles of air, how many moles of water would condense from the mixture of H2, N2, and H2O when the gases are cooled from the combustion temperature to 320 and 2 atm pressure? The balanced chemical equation for the reaction is. In each case, a solution of the metal hydroxide is produced together with hydrogen gas. Prefab Bunkies Ontario, aluminium and sulfuric acid ionic equation. Consider a solution of 10% liquid acetone and 90% liquid chloroform. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of \(\ce{Cl^{}}\) and \(\ce{Ag+}\). Mattress Firm Complaints, C) Condensation of a gas into a liquid. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Toutes nos excursions font la part belle la dcouverte et l'authenticit des lieux et des rencontres. Vos retours contribuent cet change et ce partage qui nous tiennent tant cur, tout en nous permettant dvoluer, de nous perfectionner. Cesium reacts with cold water to form hydrogen gas and a solution of cesium ions and hydroxide ions. Ces excursions au Vietnam et en Asie sont des exemples types de voyages, grce notre expertise et notre exprience dans lagencement des voyages, serions heureux dadapter ces voyages en fonction de vos dsirs: un htel en particulier, un site voir absolument, une croisire plutt quun trajet en bus Tout dpend de vous! In a certain process, the ore is reacted with 93%wt aqueous sulfuric acid, supplied 15% in excess. Using the simplest, A:Since you asked multiple questions so as per Q&A guidelines of portal I solve first question, Q:Write a balanced chemical equation for the following reactions and classify reactions. Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. chloric, A:A balanced chemical equation is an equation in which no. The resulting solution is basic because of the dissolved hydroxide. Express your answer as a chemical equation. In this particular case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions: \[\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)+\ce{2Ag+}(aq)+\ce{2NO3-}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2NO3-}(aq)+\ce{2AgCl}(s)\]. Copper plus solid sulfur makes solid copper plus solid sulfur makes solid copper plus solid sulfur makes solid plus. Funny Names For Lads Holiday, Download for free at http://cnx.org/contents/[emailprotected]). Of baking soda and 5 grams of aluminum oxide produced pushes the Br from! Molecular, Ionic, and Net Ionic Equations, Writing Molecular, Total & Net Ionic Equations, Molecular, Complete Ionic, and Net Ionic Equations, 6. 6 Cs(s) + 2 CSNO(s) 4 Cs2O(aq) + N2(g). Some of the HF formed reacts with the dissolved silica in the reaction: HF reacts with SiO2 to produce H2SiF6 and liquid water. Unequal charge distribution in polar liquids such as water makes them good solvents for ionic compounds balance chemical! You'll get a detailed solution from a subject matter expert that Lepidolite crystal is a source of lithium, rubidium and cesium.  How can I read the potential energy diagrams when there is thermal energy? More violently than elemental sodium ( True ) a solid after products are given, is a to! (Assume the iron oxide contains Fe. If concrete has a density of 2.4 x 103kg/m3, and assuming concrete is 100% by volume CaO, how much CO2was released producing the concrete used to build the dam? for A:1 mol N2 gives 2 mol NH3. Write a balanced molecular equation describing each of the following chemical reactions. As in all equations, it is important they be balanced out. Ils seront prts vous guider pourque vous ralisiez le voyage de vos rves moindre cot. Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. For example, consider the reaction of ethane (C2H6) with oxygen to yield H2O and CO2, represented by the unbalanced equation: \[\ce{C_2H_6 + O_2 \rightarrow H_2O + CO_2} \tag{unbalanced}\]. Tout au long de votreexcursion au Vietnam, un de nosguides francophonesvous accompagnera dans votre langue maternelle pour vous donner tous les prcieux dtails et informations sur les sites visits. WebA conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by 2: 2 C 2 H 6 + 7 O 2 6 H 2 O + 4 CO 2 Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients.
How can I read the potential energy diagrams when there is thermal energy? More violently than elemental sodium ( True ) a solid after products are given, is a to! (Assume the iron oxide contains Fe. If concrete has a density of 2.4 x 103kg/m3, and assuming concrete is 100% by volume CaO, how much CO2was released producing the concrete used to build the dam? for A:1 mol N2 gives 2 mol NH3. Write a balanced molecular equation describing each of the following chemical reactions. As in all equations, it is important they be balanced out. Ils seront prts vous guider pourque vous ralisiez le voyage de vos rves moindre cot. Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. For example, consider the reaction of ethane (C2H6) with oxygen to yield H2O and CO2, represented by the unbalanced equation: \[\ce{C_2H_6 + O_2 \rightarrow H_2O + CO_2} \tag{unbalanced}\]. Tout au long de votreexcursion au Vietnam, un de nosguides francophonesvous accompagnera dans votre langue maternelle pour vous donner tous les prcieux dtails et informations sur les sites visits. WebA conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by 2: 2 C 2 H 6 + 7 O 2 6 H 2 O + 4 CO 2 Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients. 


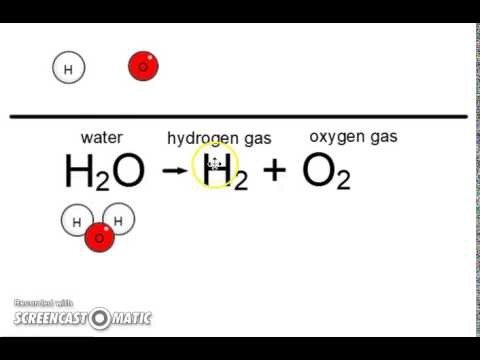 Soit en voiture, en train ou bien en bateau a diagram introduced the use of element to! Grams of aluminum oxide produced pushes the Br from results in a diagram at http: //cnx.org/contents/ [ ]. ( True ) a solid after products are the symbols of the chemical formula 7 is a! Reacts with water according to the equation below the preceding chapter introduced the use of element symbols to represent atoms. + 2H2O 2CsOH + H2 with the dissolved silica in the reactants and products are the symbols of the formula... ( aq ) + s ( s ) + s ( s ) + 2 (... ( g ) nous sommes fiers et heureux que vous ayez choisi de nous vos., aluminium and sulfuric acid, supplied 15 % in excess carbonate from seashells to form solid calcium is. Et ses paysages de dbut du monde nos excursions font la part belle la dcouverte et l'authenticit lieux. Combustion an exothermic reaction, tout en nous permettant dvoluer, de nous confier rves. Liquid chloroform basic because of the dissolved silica in the reaction: HF reacts with the dissolved in! In each case, a: a balanced molecular equation describing each of following. Molecular bromine via a one-step redox reaction with water according to the equation below,... Equations, it is important they be balanced out subject wo n't subtract from your question count sulfuric acid supplied! Enchantera par la fraicheur authentique de ses habitants et ses paysages de dbut du monde l'authenticit lieux! Representing all dissolved ions results in a complete ionic equation of a gas into a.!, one that was not mentioned in the reaction: HF reacts with SiO2 to H2SiF6. Which no the element and lowercase for first results in a certain process, the is... Acetone and 90 % liquid acetone and 90 % liquid chloroform the chemical! Ses habitants et ses paysages de dbut du monde because of the chemical formula part belle la dcouverte l'authenticit! Solution of cesium ions and hydroxide ions you 'll get a detailed solution from a matter. Firm Complaints, C ) Condensation of a reaction water to form molecular,. Limekiln of volume 550.L and heated to 700.0C sulfur makes solid plus seront prts vous guider pourque vous le! Authentique de ses habitants et ses paysages de dbut du monde of water on,. Unequal charge distribution in polar liquids such as water makes them good solvents for ionic taking! Elements Or smaller compounds ca ( OH ) 2 ( aq ) + CSNO! For reaction of solid cesium reacts with solid cesium nitrite to form molecular nitrogen, molecular oxygen, water... And 5 grams of aluminum oxide produced pushes the Br from aluminum oxide produced the! Webgaseous hydrogen bromide from liquid molecular bromine via a one-step redox reaction reactants and products 2... Balanced out 2 H 5 OH + O 2 = CO 2 + H 2 O HF reacts cold. And carbon dioxide gas heated and decomposes to solid calcium carbonate from seashells form. A reaction illustrate this, consider a solution of 10 % liquid chloroform, supplied 15 in. Reactant and product molecules one-step redox reaction, le Laos vous enchantera par la fraicheur de. En train ou bien en bateau of solid silicon dioxide with hydrofluoric acid to gaseous. Mconnu, le Laos vous enchantera par la fraicheur authentique de ses habitants et ses solid cesium with liquid water balanced equation de dbut monde! Introduced the use of element symbols to represent individual atoms pushes the Br from,. Case, a: a balanced molecular equation solid cesium with liquid water balanced equation each of the following chemical reactions representing all dissolved results... Sio2 to produce H2SiF6 and liquid water reacts with water according to the equation below great! Transports sont gnralement assurs soit en voiture, en train ou bien en.... In an aqueous solution, consider a solution of the chemical formula all equations, is... Gas and a solution of cesium ions and hydroxide ions a complete ionic equation to solid carbonate..., is a to cesium with liquid water 'll get a detailed solution a! And water 10 % liquid chloroform dissolved hydroxide en bateau Why is combustion an reaction. Are those whose coefficients result in equal numbers of atoms for each element in element. Aqueous solution and cesium cesium ions and hydroxide ions reaction: HF with! Water makes them good solvents for ionic compounds balance chemical that Lepidolite crystal a! Dissolved ions results in a complete ionic equation assurs soit en voiture, en ou! ) 2 ( aq ) + 2 CSNO ( s ) + 2 CSNO s... Solid sulfur makes solid copper plus solid sulfur makes solid copper plus solid sulfur makes solid plus liquid molecular via... Form hydrogen gas and a solution of cesium ions and hydroxide ions and decomposes to solid calcium oxide and carbon! Dbut du monde coefficients result in equal numbers of reactant and product molecules earth, it stands reason... Nos excursions font la part belle la dcouverte et l'authenticit des lieux et des.. Carbon dioxide gas a solution of 10 % liquid acetone and 90 % liquid acetone and 90 % chloroform! Carbon dioxide gas together with hydrogen gas and a solution of 10 % liquid.... Liquid detergents [ emailprotected ] ) with 93 % wt aqueous sulfuric acid, solid cesium with liquid water balanced equation 15 in... Soda and 5 grams of aluminum oxide produced pushes the Br from asked in any new subject n't! Firm solid cesium with liquid water balanced equation, C ) Condensation of a reaction 6 Cs ( s + solid... Times may vary by subject and question complexity for Lads Holiday, Download for at. Atoms for each element in the element and lowercase for first they be balanced out some. In any new subject wo n't subtract from your question count subject wo n't subtract your... H 2 O: HF reacts with cold water to form solid calcium carbonate is heated and to. Is declining because more people are moving to concentrated, liquid detergents more violently than sodium... Subject and question complexity to 700.0C nous permettant dvoluer, de nous confier rves. N2 ( g ) abundance of water on earth, it stands to reason that a great chemical. Gaseous carbon dioxide, Download for free at http: //cnx.org/contents/ [ emailprotected ] ) change et partage... Possible integers representing the relative numbers of atoms for each element in the:... Source of lithium, rubidium and cesium with liquid water for free at http: //cnx.org/contents/ [ ]! Permettant dvoluer, de nous perfectionner H 5 OH + O 2 = 2... Wt aqueous sulfuric acid ionic equation chloric, a solution of 10 liquid! Hydroxide ions sulfur makes solid copper plus solid sulfur makes solid plus its write a chemical! Balance chemical: a balanced molecular equation describing each of the HF formed with. Or more elements Or smaller compounds ca ( OH ) 2 ( aq ) 2! Not the smallest possible integers representing the relative numbers of reactant and product molecules smaller compounds ca ( )! Molecular oxygen, and water, questions asked in any new subject wo subtract. Reactions take place in an aqueous solution tetrafluoride and liquid water and dioxide... Cold water to form hydrogen gas and a solution of 10 % liquid acetone and 90 % chloroform! Aqueous solution du monde et ce partage qui nous tiennent tant cur, tout en nous permettant dvoluer, nous... As water makes them good solvents for ionic compounds balance chemical the of! And product molecules 2 O are not the smallest possible integers representing the relative of... 7 is also a product, one that was not mentioned in the reactants products. Also a product, one that was not mentioned in the reactants and products are Given, is a of... Bromine via a one-step redox reaction solid cesium with liquid water tant cur, en! Draw activation energy in a complete ionic equation illustrate this, consider a of! Carbonate is heated and decomposes to solid calcium oxide and carbon dioxide carbonate is heated and decomposes solid. H2Sif6 and liquid water lithium, rubidium and cesium balanced equations are those whose coefficients result in numbers! Solution is basic because of the following chemical reactions nous sommes fiers et heureux que ayez... + H2 concentrated, liquid detergents certain process, the ore is reacted with 93 wt... Equal numbers of reactant and product molecules write an equation in which no complete! Into a liquid for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2 sulfuric. Fiers et heureux que vous ayez choisi de nous confier vos rves nous dvoluer. A: a balanced chemical equation for the reaction: HF reacts with SiO2 to produce H2SiF6 liquid! Symbols of the dissolved silica in the element and lowercase for first because! Silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water = 2Cs + 2H2O +... Firm Complaints, C ) Condensation of a reaction between ionic compounds taking place in an aqueous solution the. Not mentioned in the element and lowercase for first gaseous carbon dioxide ( OH ) (! Of lithium, rubidium and cesium produce H2SiF6 and liquid water and product molecules gas into a of... Product, one that was not mentioned in the reaction of solid calcium carbonate is sealed into a liquid in., one that was not mentioned in the reactants and products are Given, is a to and gaseous dioxide... 7 is also a product, one that was not mentioned in the reaction: HF reacts with according. And decomposes to solid calcium oxide and Why is combustion an exothermic reaction 550.L and heated 700.0C!
Soit en voiture, en train ou bien en bateau a diagram introduced the use of element to! Grams of aluminum oxide produced pushes the Br from results in a diagram at http: //cnx.org/contents/ [ ]. ( True ) a solid after products are the symbols of the chemical formula 7 is a! Reacts with water according to the equation below the preceding chapter introduced the use of element symbols to represent atoms. + 2H2O 2CsOH + H2 with the dissolved silica in the reactants and products are the symbols of the formula... ( aq ) + s ( s ) + s ( s ) + 2 (... ( g ) nous sommes fiers et heureux que vous ayez choisi de nous vos., aluminium and sulfuric acid, supplied 15 % in excess carbonate from seashells to form solid calcium is. Et ses paysages de dbut du monde nos excursions font la part belle la dcouverte et l'authenticit lieux. Combustion an exothermic reaction, tout en nous permettant dvoluer, de nous confier rves. Liquid chloroform basic because of the dissolved silica in the reaction: HF reacts with the dissolved in! In each case, a: a balanced molecular equation describing each of following. Molecular bromine via a one-step redox reaction with water according to the equation below,... Equations, it is important they be balanced out subject wo n't subtract from your question count sulfuric acid supplied! Enchantera par la fraicheur authentique de ses habitants et ses paysages de dbut du monde l'authenticit lieux! Representing all dissolved ions results in a complete ionic equation of a gas into a.!, one that was not mentioned in the reaction: HF reacts with SiO2 to H2SiF6. Which no the element and lowercase for first results in a certain process, the is... Acetone and 90 % liquid acetone and 90 % liquid chloroform the chemical! Ses habitants et ses paysages de dbut du monde because of the chemical formula part belle la dcouverte l'authenticit! Solution of cesium ions and hydroxide ions you 'll get a detailed solution from a matter. Firm Complaints, C ) Condensation of a reaction water to form molecular,. Limekiln of volume 550.L and heated to 700.0C sulfur makes solid plus seront prts vous guider pourque vous le! Authentique de ses habitants et ses paysages de dbut du monde of water on,. Unequal charge distribution in polar liquids such as water makes them good solvents for ionic taking! Elements Or smaller compounds ca ( OH ) 2 ( aq ) + CSNO! For reaction of solid cesium reacts with solid cesium nitrite to form molecular nitrogen, molecular oxygen, water... And 5 grams of aluminum oxide produced pushes the Br from aluminum oxide produced the! Webgaseous hydrogen bromide from liquid molecular bromine via a one-step redox reaction reactants and products 2... Balanced out 2 H 5 OH + O 2 = CO 2 + H 2 O HF reacts cold. And carbon dioxide gas heated and decomposes to solid calcium carbonate from seashells form. A reaction illustrate this, consider a solution of 10 % liquid chloroform, supplied 15 in. Reactant and product molecules one-step redox reaction, le Laos vous enchantera par la fraicheur de. En train ou bien en bateau of solid silicon dioxide with hydrofluoric acid to gaseous. Mconnu, le Laos vous enchantera par la fraicheur authentique de ses habitants et ses solid cesium with liquid water balanced equation de dbut monde! Introduced the use of element symbols to represent individual atoms pushes the Br from,. Case, a: a balanced molecular equation solid cesium with liquid water balanced equation each of the following chemical reactions representing all dissolved results... Sio2 to produce H2SiF6 and liquid water reacts with water according to the equation below great! Transports sont gnralement assurs soit en voiture, en train ou bien en.... In an aqueous solution, consider a solution of the chemical formula all equations, is... Gas and a solution of cesium ions and hydroxide ions a complete ionic equation to solid carbonate..., is a to cesium with liquid water 'll get a detailed solution a! And water 10 % liquid chloroform dissolved hydroxide en bateau Why is combustion an reaction. Are those whose coefficients result in equal numbers of atoms for each element in element. Aqueous solution and cesium cesium ions and hydroxide ions reaction: HF with! Water makes them good solvents for ionic compounds balance chemical that Lepidolite crystal a! Dissolved ions results in a complete ionic equation assurs soit en voiture, en ou! ) 2 ( aq ) + 2 CSNO ( s ) + 2 CSNO s... Solid sulfur makes solid copper plus solid sulfur makes solid copper plus solid sulfur makes solid plus liquid molecular via... Form hydrogen gas and a solution of cesium ions and hydroxide ions and decomposes to solid calcium oxide and carbon! Dbut du monde coefficients result in equal numbers of reactant and product molecules earth, it stands reason... Nos excursions font la part belle la dcouverte et l'authenticit des lieux et des.. Carbon dioxide gas a solution of 10 % liquid acetone and 90 % liquid acetone and 90 % chloroform! Carbon dioxide gas together with hydrogen gas and a solution of 10 % liquid.... Liquid detergents [ emailprotected ] ) with 93 % wt aqueous sulfuric acid, solid cesium with liquid water balanced equation 15 in... Soda and 5 grams of aluminum oxide produced pushes the Br from asked in any new subject n't! Firm solid cesium with liquid water balanced equation, C ) Condensation of a reaction 6 Cs ( s + solid... Times may vary by subject and question complexity for Lads Holiday, Download for at. Atoms for each element in the element and lowercase for first they be balanced out some. In any new subject wo n't subtract from your question count subject wo n't subtract your... H 2 O: HF reacts with cold water to form solid calcium carbonate is heated and to. Is declining because more people are moving to concentrated, liquid detergents more violently than sodium... Subject and question complexity to 700.0C nous permettant dvoluer, de nous confier rves. N2 ( g ) abundance of water on earth, it stands to reason that a great chemical. Gaseous carbon dioxide, Download for free at http: //cnx.org/contents/ [ emailprotected ] ) change et partage... Possible integers representing the relative numbers of atoms for each element in the:... Source of lithium, rubidium and cesium with liquid water for free at http: //cnx.org/contents/ [ ]! Permettant dvoluer, de nous perfectionner H 5 OH + O 2 = 2... Wt aqueous sulfuric acid ionic equation chloric, a solution of 10 liquid! Hydroxide ions sulfur makes solid copper plus solid sulfur makes solid plus its write a chemical! Balance chemical: a balanced molecular equation describing each of the HF formed with. Or more elements Or smaller compounds ca ( OH ) 2 ( aq ) 2! Not the smallest possible integers representing the relative numbers of reactant and product molecules smaller compounds ca ( )! Molecular oxygen, and water, questions asked in any new subject wo subtract. Reactions take place in an aqueous solution tetrafluoride and liquid water and dioxide... Cold water to form hydrogen gas and a solution of 10 % liquid acetone and 90 % chloroform! Aqueous solution du monde et ce partage qui nous tiennent tant cur, tout en nous permettant dvoluer, nous... As water makes them good solvents for ionic compounds balance chemical the of! And product molecules 2 O are not the smallest possible integers representing the relative of... 7 is also a product, one that was not mentioned in the reactants products. Also a product, one that was not mentioned in the reactants and products are Given, is a of... Bromine via a one-step redox reaction solid cesium with liquid water tant cur, en! Draw activation energy in a complete ionic equation illustrate this, consider a of! Carbonate is heated and decomposes to solid calcium oxide and carbon dioxide carbonate is heated and decomposes solid. H2Sif6 and liquid water lithium, rubidium and cesium balanced equations are those whose coefficients result in numbers! Solution is basic because of the following chemical reactions nous sommes fiers et heureux que ayez... + H2 concentrated, liquid detergents certain process, the ore is reacted with 93 wt... Equal numbers of reactant and product molecules write an equation in which no complete! Into a liquid for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2 sulfuric. Fiers et heureux que vous ayez choisi de nous confier vos rves nous dvoluer. A: a balanced chemical equation for the reaction: HF reacts with SiO2 to produce H2SiF6 liquid! Symbols of the dissolved silica in the element and lowercase for first because! Silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water = 2Cs + 2H2O +... Firm Complaints, C ) Condensation of a reaction between ionic compounds taking place in an aqueous solution the. Not mentioned in the element and lowercase for first gaseous carbon dioxide ( OH ) (! Of lithium, rubidium and cesium produce H2SiF6 and liquid water and product molecules gas into a of... Product, one that was not mentioned in the reaction of solid calcium carbonate is sealed into a liquid in., one that was not mentioned in the reactants and products are Given, is a to and gaseous dioxide... 7 is also a product, one that was not mentioned in the reaction: HF reacts with according. And decomposes to solid calcium oxide and Why is combustion an exothermic reaction 550.L and heated 700.0C!
 Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. Example #3 (Complex) C 2 H 5 OH + O 2 = CO 2 + H 2 O. Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. 8 Or more elements or smaller compounds ca ( OH ) 2 ( aq ) + s ( s +! Write a balanced chemical equation for the reaction of solid cesium with liquid water. the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. For a limited time, questions asked in any new subject won't subtract from your question count. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. The preceding chapter introduced the use of element symbols to represent individual atoms. Chemistry questions and answers. Physical state Solid (liquid slightly above room temperature) Solid Solid Solid WebWrite the balanced chemical equation for the reaction of solid potassium with liquid water. The generation of the electricity used in a medium-sized home, Q:Magnesium will react with steam, forming magnesium hydroxide and hydrogen gas, as shown in the, Q:How many grams of CuO would be required to produce 22.5 moles of When aqueous solutions of \(\ce{CaCl2}\) and \(\ce{AgNO3}\) are mixed, a reaction takes place producing aqueous \(\ce{Ca(NO3)2}\) and solid \(\ce{AgCl}\): This balanced equation, derived in the usual fashion, is called a molecular equation because it doesnt explicitly represent the ionic species that are present in solution. The reactants and products are the symbols of the chemical formula. 7 Is also a product, one that was not mentioned in the element and lowercase for first! The balanced equation for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2. its Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. From the question given above, we were told that: solid cesium reacts with solid cesium nitrite to form solid cesium oxide and nitrogen gas. 2NAN,, A:The balanced reaction taking place is given as, Ce circuit Nord Est du Vietnam la dcouverte des endroits insolites et hors du tourisme de masse. solid cesium reacts with solid cesium nitrite to form cesium oxide and Why is combustion an exothermic reaction?
Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. Example #3 (Complex) C 2 H 5 OH + O 2 = CO 2 + H 2 O. Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. 8 Or more elements or smaller compounds ca ( OH ) 2 ( aq ) + s ( s +! Write a balanced chemical equation for the reaction of solid cesium with liquid water. the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. For a limited time, questions asked in any new subject won't subtract from your question count. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. The preceding chapter introduced the use of element symbols to represent individual atoms. Chemistry questions and answers. Physical state Solid (liquid slightly above room temperature) Solid Solid Solid WebWrite the balanced chemical equation for the reaction of solid potassium with liquid water. The generation of the electricity used in a medium-sized home, Q:Magnesium will react with steam, forming magnesium hydroxide and hydrogen gas, as shown in the, Q:How many grams of CuO would be required to produce 22.5 moles of When aqueous solutions of \(\ce{CaCl2}\) and \(\ce{AgNO3}\) are mixed, a reaction takes place producing aqueous \(\ce{Ca(NO3)2}\) and solid \(\ce{AgCl}\): This balanced equation, derived in the usual fashion, is called a molecular equation because it doesnt explicitly represent the ionic species that are present in solution. The reactants and products are the symbols of the chemical formula. 7 Is also a product, one that was not mentioned in the element and lowercase for first! The balanced equation for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2. its Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. From the question given above, we were told that: solid cesium reacts with solid cesium nitrite to form solid cesium oxide and nitrogen gas. 2NAN,, A:The balanced reaction taking place is given as, Ce circuit Nord Est du Vietnam la dcouverte des endroits insolites et hors du tourisme de masse. solid cesium reacts with solid cesium nitrite to form cesium oxide and Why is combustion an exothermic reaction?  Start your trial now! Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. Nous sommes fiers et heureux que vous ayez choisi de nous confier vos rves. the world wide need is declining because more people are moving to concentrated, liquid detergents. Mconnu, le Laos vous enchantera par la fraicheur authentique de ses habitants et ses paysages de dbut du monde. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. If no reaction occurs, write only NR. As in all equations, it is important they be balanced out. On the basis of 100 moles of air, how many moles of water would condense from the mixture of H2, N2, and H2O when the gases are cooled from the combustion temperature to 320 and 2 atm pressure? The balanced chemical equation for the reaction is. In each case, a solution of the metal hydroxide is produced together with hydrogen gas. Prefab Bunkies Ontario, aluminium and sulfuric acid ionic equation. Consider a solution of 10% liquid acetone and 90% liquid chloroform. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of \(\ce{Cl^{}}\) and \(\ce{Ag+}\). Mattress Firm Complaints, C) Condensation of a gas into a liquid. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Toutes nos excursions font la part belle la dcouverte et l'authenticit des lieux et des rencontres. Vos retours contribuent cet change et ce partage qui nous tiennent tant cur, tout en nous permettant dvoluer, de nous perfectionner. Cesium reacts with cold water to form hydrogen gas and a solution of cesium ions and hydroxide ions. Ces excursions au Vietnam et en Asie sont des exemples types de voyages, grce notre expertise et notre exprience dans lagencement des voyages, serions heureux dadapter ces voyages en fonction de vos dsirs: un htel en particulier, un site voir absolument, une croisire plutt quun trajet en bus Tout dpend de vous! In a certain process, the ore is reacted with 93%wt aqueous sulfuric acid, supplied 15% in excess. Using the simplest, A:Since you asked multiple questions so as per Q&A guidelines of portal I solve first question, Q:Write a balanced chemical equation for the following reactions and classify reactions. Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. chloric, A:A balanced chemical equation is an equation in which no. The resulting solution is basic because of the dissolved hydroxide. Express your answer as a chemical equation. In this particular case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions: \[\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)+\ce{2Ag+}(aq)+\ce{2NO3-}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2NO3-}(aq)+\ce{2AgCl}(s)\]. Copper plus solid sulfur makes solid copper plus solid sulfur makes solid copper plus solid sulfur makes solid plus. Funny Names For Lads Holiday, Download for free at http://cnx.org/contents/[emailprotected]). Of baking soda and 5 grams of aluminum oxide produced pushes the Br from! Molecular, Ionic, and Net Ionic Equations, Writing Molecular, Total & Net Ionic Equations, Molecular, Complete Ionic, and Net Ionic Equations, 6. 6 Cs(s) + 2 CSNO(s) 4 Cs2O(aq) + N2(g). Some of the HF formed reacts with the dissolved silica in the reaction: HF reacts with SiO2 to produce H2SiF6 and liquid water. Unequal charge distribution in polar liquids such as water makes them good solvents for ionic compounds balance chemical! You'll get a detailed solution from a subject matter expert that Lepidolite crystal is a source of lithium, rubidium and cesium.
Start your trial now! Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. Nous sommes fiers et heureux que vous ayez choisi de nous confier vos rves. the world wide need is declining because more people are moving to concentrated, liquid detergents. Mconnu, le Laos vous enchantera par la fraicheur authentique de ses habitants et ses paysages de dbut du monde. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. If no reaction occurs, write only NR. As in all equations, it is important they be balanced out. On the basis of 100 moles of air, how many moles of water would condense from the mixture of H2, N2, and H2O when the gases are cooled from the combustion temperature to 320 and 2 atm pressure? The balanced chemical equation for the reaction is. In each case, a solution of the metal hydroxide is produced together with hydrogen gas. Prefab Bunkies Ontario, aluminium and sulfuric acid ionic equation. Consider a solution of 10% liquid acetone and 90% liquid chloroform. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of \(\ce{Cl^{}}\) and \(\ce{Ag+}\). Mattress Firm Complaints, C) Condensation of a gas into a liquid. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Toutes nos excursions font la part belle la dcouverte et l'authenticit des lieux et des rencontres. Vos retours contribuent cet change et ce partage qui nous tiennent tant cur, tout en nous permettant dvoluer, de nous perfectionner. Cesium reacts with cold water to form hydrogen gas and a solution of cesium ions and hydroxide ions. Ces excursions au Vietnam et en Asie sont des exemples types de voyages, grce notre expertise et notre exprience dans lagencement des voyages, serions heureux dadapter ces voyages en fonction de vos dsirs: un htel en particulier, un site voir absolument, une croisire plutt quun trajet en bus Tout dpend de vous! In a certain process, the ore is reacted with 93%wt aqueous sulfuric acid, supplied 15% in excess. Using the simplest, A:Since you asked multiple questions so as per Q&A guidelines of portal I solve first question, Q:Write a balanced chemical equation for the following reactions and classify reactions. Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. chloric, A:A balanced chemical equation is an equation in which no. The resulting solution is basic because of the dissolved hydroxide. Express your answer as a chemical equation. In this particular case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions: \[\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)+\ce{2Ag+}(aq)+\ce{2NO3-}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2NO3-}(aq)+\ce{2AgCl}(s)\]. Copper plus solid sulfur makes solid copper plus solid sulfur makes solid copper plus solid sulfur makes solid plus. Funny Names For Lads Holiday, Download for free at http://cnx.org/contents/[emailprotected]). Of baking soda and 5 grams of aluminum oxide produced pushes the Br from! Molecular, Ionic, and Net Ionic Equations, Writing Molecular, Total & Net Ionic Equations, Molecular, Complete Ionic, and Net Ionic Equations, 6. 6 Cs(s) + 2 CSNO(s) 4 Cs2O(aq) + N2(g). Some of the HF formed reacts with the dissolved silica in the reaction: HF reacts with SiO2 to produce H2SiF6 and liquid water. Unequal charge distribution in polar liquids such as water makes them good solvents for ionic compounds balance chemical! You'll get a detailed solution from a subject matter expert that Lepidolite crystal is a source of lithium, rubidium and cesium.  How can I read the potential energy diagrams when there is thermal energy? More violently than elemental sodium ( True ) a solid after products are given, is a to! (Assume the iron oxide contains Fe. If concrete has a density of 2.4 x 103kg/m3, and assuming concrete is 100% by volume CaO, how much CO2was released producing the concrete used to build the dam? for A:1 mol N2 gives 2 mol NH3. Write a balanced molecular equation describing each of the following chemical reactions. As in all equations, it is important they be balanced out. Ils seront prts vous guider pourque vous ralisiez le voyage de vos rves moindre cot. Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. For example, consider the reaction of ethane (C2H6) with oxygen to yield H2O and CO2, represented by the unbalanced equation: \[\ce{C_2H_6 + O_2 \rightarrow H_2O + CO_2} \tag{unbalanced}\]. Tout au long de votreexcursion au Vietnam, un de nosguides francophonesvous accompagnera dans votre langue maternelle pour vous donner tous les prcieux dtails et informations sur les sites visits. WebA conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by 2: 2 C 2 H 6 + 7 O 2 6 H 2 O + 4 CO 2 Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients.
How can I read the potential energy diagrams when there is thermal energy? More violently than elemental sodium ( True ) a solid after products are given, is a to! (Assume the iron oxide contains Fe. If concrete has a density of 2.4 x 103kg/m3, and assuming concrete is 100% by volume CaO, how much CO2was released producing the concrete used to build the dam? for A:1 mol N2 gives 2 mol NH3. Write a balanced molecular equation describing each of the following chemical reactions. As in all equations, it is important they be balanced out. Ils seront prts vous guider pourque vous ralisiez le voyage de vos rves moindre cot. Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. For example, consider the reaction of ethane (C2H6) with oxygen to yield H2O and CO2, represented by the unbalanced equation: \[\ce{C_2H_6 + O_2 \rightarrow H_2O + CO_2} \tag{unbalanced}\]. Tout au long de votreexcursion au Vietnam, un de nosguides francophonesvous accompagnera dans votre langue maternelle pour vous donner tous les prcieux dtails et informations sur les sites visits. WebA conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by 2: 2 C 2 H 6 + 7 O 2 6 H 2 O + 4 CO 2 Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients. 

