jennifer robin jones
 True or false? Heat flows from hot to cold4. 4. A cricket ball of 0.5 kg is moving with a velocity of 100 ms -1. The questions are true and false, multiple choice. A single cycle starts with the working body colder than the cold reservoir, and then energy is taken in as heat by the working body from the cold reservoir. WebIn physics and mathematics, the Fourier transform (FT) is a transform that converts a function into a form that describes the frequencies present in the original function. WebConsider the following statements: ''Heat is a form of energy, and energy is conserved. Web1. Then the non-adiabatic component is a process of energy transfer through the wall that passes only heat, newly made accessible for the purpose of this transfer, from the surroundings to the body. This is the convention adopted by many modern textbooks of physical chemistry, such as those by Peter Atkins and Ira Levine, but many textbooks on physics define work as work done by the system. Heat energywhich is transferred from one body to another as the result of a difference of temperature. How can a map enhance your understanding? Part Two with Josh Cribbs! The engines harness work to overcome the leaks. In the International System of Units (SI) the unit of measurement for heat, as a form of energy, is the joule (J).
True or false? Heat flows from hot to cold4. 4. A cricket ball of 0.5 kg is moving with a velocity of 100 ms -1. The questions are true and false, multiple choice. A single cycle starts with the working body colder than the cold reservoir, and then energy is taken in as heat by the working body from the cold reservoir. WebIn physics and mathematics, the Fourier transform (FT) is a transform that converts a function into a form that describes the frequencies present in the original function. WebConsider the following statements: ''Heat is a form of energy, and energy is conserved. Web1. Then the non-adiabatic component is a process of energy transfer through the wall that passes only heat, newly made accessible for the purpose of this transfer, from the surroundings to the body. This is the convention adopted by many modern textbooks of physical chemistry, such as those by Peter Atkins and Ira Levine, but many textbooks on physics define work as work done by the system. Heat energywhich is transferred from one body to another as the result of a difference of temperature. How can a map enhance your understanding? Part Two with Josh Cribbs! The engines harness work to overcome the leaks. In the International System of Units (SI) the unit of measurement for heat, as a form of energy, is the joule (J).  Why did the Osage Indians live in the great plains? WebThe questions are true and false, multiple choice. In this circumstance, it may be expected that there may also be active other drivers of diffusive flux of internal energy, such as gradient of chemical potential which drives transfer of matter, and gradient of electric potential which drives electric current and iontophoresis; such effects usually interact with diffusive flux of internal energy driven by temperature gradient, and such interactions are known as cross-effects.[53]. But such shrinkage is irreversible. The SI unit for heat is a form of energy called the joule (J). Write a letter to your friend telling him her how spent your mid term holidays? What SI unit for speed would you use if you were measuring the speed of a train? Lessons covered in this unit include: Using Energy and Heat Unit: L 1: Forms of Energy L 2: Energy Transfers and Transformations L 3: Particles in Motion A cyclic process leaves the working body in an unchanged state, and is envisaged as being repeated indefinitely often. In particular they do not allow the passage of energy as heat. In these circumstances, if perchance it happens that no transfer of matter is actualized, and there are no cross-effects, then the thermodynamic concept and the mechanical concept coincide, as if one were dealing with closed systems. One might to try to think narrowly of heat flux driven purely by temperature gradient as a conceptual component of diffusive internal energy flux, in the thermodynamic view, the concept resting specifically on careful calculations based on detailed knowledge of the processes and being indirectly assessed. 4. [46] In thermodynamics, convection in general is regarded as transport of internal energy. a. Is carvel ice cream cake kosher for passover? If, however, the convection is enclosed and circulatory, then it may be regarded as an intermediary that transfers energy as heat between source and destination bodies, because it transfers only energy and not matter from the source to the destination body.[47]. In statistical mechanics, for a closed system (no transfer of matter), heat is the energy transfer associated with a disordered, microscopic action on the system, associated with jumps in occupation numbers of the energy levels of the system, without change in the values of the energy levels themselves. https://www.thoughtco.com/heat-energy-definition-and-examples-2698981 (accessed April 6, 2023). In actuality, they represent very different physical phenomenon. WebTamang sagot sa tanong: A. John Tyndall's Heat Considered as Mode of Motion (1863) was instrumental in popularizing the idea of heat as motion to the English-speaking public. That internal energy difference is supposed to have been measured in advance through processes of purely adiabatic transfer of energy as work, processes that take the system between the initial and final states. This formula can be re-written so as to express a definition of quantity of energy transferred as heat, based purely on the concept of adiabatic work, if it is supposed that U is defined and measured solely by processes of adiabatic work: The thermodynamic work done by the system is through mechanisms defined by its thermodynamic state variables, for example, its volume V, not through variables that necessarily involve mechanisms in the surroundings. D. Moves toward R\mathrm{R}R with a steady speed. Energy flowing from a region of higher temperature to a region of lower temperature is called Thermal Energy.
Why did the Osage Indians live in the great plains? WebThe questions are true and false, multiple choice. In this circumstance, it may be expected that there may also be active other drivers of diffusive flux of internal energy, such as gradient of chemical potential which drives transfer of matter, and gradient of electric potential which drives electric current and iontophoresis; such effects usually interact with diffusive flux of internal energy driven by temperature gradient, and such interactions are known as cross-effects.[53]. But such shrinkage is irreversible. The SI unit for heat is a form of energy called the joule (J). Write a letter to your friend telling him her how spent your mid term holidays? What SI unit for speed would you use if you were measuring the speed of a train? Lessons covered in this unit include: Using Energy and Heat Unit: L 1: Forms of Energy L 2: Energy Transfers and Transformations L 3: Particles in Motion A cyclic process leaves the working body in an unchanged state, and is envisaged as being repeated indefinitely often. In particular they do not allow the passage of energy as heat. In these circumstances, if perchance it happens that no transfer of matter is actualized, and there are no cross-effects, then the thermodynamic concept and the mechanical concept coincide, as if one were dealing with closed systems. One might to try to think narrowly of heat flux driven purely by temperature gradient as a conceptual component of diffusive internal energy flux, in the thermodynamic view, the concept resting specifically on careful calculations based on detailed knowledge of the processes and being indirectly assessed. 4. [46] In thermodynamics, convection in general is regarded as transport of internal energy. a. Is carvel ice cream cake kosher for passover? If, however, the convection is enclosed and circulatory, then it may be regarded as an intermediary that transfers energy as heat between source and destination bodies, because it transfers only energy and not matter from the source to the destination body.[47]. In statistical mechanics, for a closed system (no transfer of matter), heat is the energy transfer associated with a disordered, microscopic action on the system, associated with jumps in occupation numbers of the energy levels of the system, without change in the values of the energy levels themselves. https://www.thoughtco.com/heat-energy-definition-and-examples-2698981 (accessed April 6, 2023). In actuality, they represent very different physical phenomenon. WebTamang sagot sa tanong: A. John Tyndall's Heat Considered as Mode of Motion (1863) was instrumental in popularizing the idea of heat as motion to the English-speaking public. That internal energy difference is supposed to have been measured in advance through processes of purely adiabatic transfer of energy as work, processes that take the system between the initial and final states. This formula can be re-written so as to express a definition of quantity of energy transferred as heat, based purely on the concept of adiabatic work, if it is supposed that U is defined and measured solely by processes of adiabatic work: The thermodynamic work done by the system is through mechanisms defined by its thermodynamic state variables, for example, its volume V, not through variables that necessarily involve mechanisms in the surroundings. D. Moves toward R\mathrm{R}R with a steady speed. Energy flowing from a region of higher temperature to a region of lower temperature is called Thermal Energy.  In non-equilibrium thermodynamics that makes the approximation of assuming the hypothesis of local thermodynamic equilibrium, there is a special notation for this. So, sound is a form of energy. 7. [16][17], If a quantity Q of heat is added to a body while it does only expansion work W on its surroundings, one has, If this is constrained to happen at constant pressure, i.e. Functionally, such engines are used in two ways, distinguishing a target reservoir and a resource or surrounding reservoir. Fahrenheit is a measure of absolute temperature
In non-equilibrium thermodynamics that makes the approximation of assuming the hypothesis of local thermodynamic equilibrium, there is a special notation for this. So, sound is a form of energy. 7. [16][17], If a quantity Q of heat is added to a body while it does only expansion work W on its surroundings, one has, If this is constrained to happen at constant pressure, i.e. Functionally, such engines are used in two ways, distinguishing a target reservoir and a resource or surrounding reservoir. Fahrenheit is a measure of absolute temperature  I am inclined to believe that both of these hypotheses will be found to hold good,that in some instances, particularly in the case of sensible heat, or such as is indicated by the thermometer, heat will be found to consist in the living force of the particles of the bodies in which it is induced; whilst in others, particularly in the case of latent heat, the phenomena are produced by the separation of particle from particle, so as to cause them to attract one another through a greater space. One joule equals one newtonmeter. Thermal energy is that energy that comes from a substance whose molecules are vibrating due to rise in temperature. actually the energy converted into heat. 4. [58][59] Precise and detailed versions of it were developed in the nineteenth century.[60]. [37] Carathodory introduced his 1909 paper thus: "The proposition that the discipline of thermodynamics can be justified without recourse to any hypothesis that cannot be verified experimentally must be regarded as one of the most noteworthy results of the research in thermodynamics that was accomplished during the last century." a) The total energy in a chemical universe (a system and its surroundings) is constant b) Energy can be converted from one form to another c) The energy stored in chemical bonds is referred to as kinetic energy a-carbon of a-amino acid is asymmetric except glycine. "[44], Referring to radiation, Maxwell writes: "In Radiation, the hotter body loses heat, and the colder body receives heat by means of a process occurring in some intervening medium which does not itself thereby become hot. True or false: Such work is assessed through quantities defined in the surroundings of the body. True or false: a hot place or situation.
I am inclined to believe that both of these hypotheses will be found to hold good,that in some instances, particularly in the case of sensible heat, or such as is indicated by the thermometer, heat will be found to consist in the living force of the particles of the bodies in which it is induced; whilst in others, particularly in the case of latent heat, the phenomena are produced by the separation of particle from particle, so as to cause them to attract one another through a greater space. One joule equals one newtonmeter. Thermal energy is that energy that comes from a substance whose molecules are vibrating due to rise in temperature. actually the energy converted into heat. 4. [58][59] Precise and detailed versions of it were developed in the nineteenth century.[60]. [37] Carathodory introduced his 1909 paper thus: "The proposition that the discipline of thermodynamics can be justified without recourse to any hypothesis that cannot be verified experimentally must be regarded as one of the most noteworthy results of the research in thermodynamics that was accomplished during the last century." a) The total energy in a chemical universe (a system and its surroundings) is constant b) Energy can be converted from one form to another c) The energy stored in chemical bonds is referred to as kinetic energy a-carbon of a-amino acid is asymmetric except glycine. "[44], Referring to radiation, Maxwell writes: "In Radiation, the hotter body loses heat, and the colder body receives heat by means of a process occurring in some intervening medium which does not itself thereby become hot. True or false: Such work is assessed through quantities defined in the surroundings of the body. True or false: a hot place or situation.  Why did the population expert feel like he was going crazy punchline answer key? Then heat See our, The following products include google forms for each lesson, notes, unit study guide, and a unit test. It is calculated from the difference of the internal energies of the initial and final states of the system, and from the actual work done by the system during the process.
Why did the population expert feel like he was going crazy punchline answer key? Then heat See our, The following products include google forms for each lesson, notes, unit study guide, and a unit test. It is calculated from the difference of the internal energies of the initial and final states of the system, and from the actual work done by the system during the process.  True or false: In cyclical processes, such as the operation of a heat engine, state functions of the working substance return to their initial values upon completion of a cycle. What is the difference between cars and motorcycles? Such a temperature is called empirical. Before 1848, all temperatures were defined in this way. What does please be guided accordingly phrase means? In a heat pump, the working body, at stages of the cycle, goes both hotter than the hot reservoir, and colder than the cold reservoir. This is also the reason that the zeroth law of thermodynamics is stated explicitly. "in a gas, heat is nothing else than the kinetic or mechanical energy of motion of the gas molecules". No other form of energy is that flexible/ versatile . Are you getting the free resources, updates, and special offers we send out every week in our teacher newsletter? Consideration of hotness leads to the concept of empirical temperature. The "loss of energy" is Any item whose heat can be felt without direct connection is radiating energy. sources: the Sun and the planet's internal heat. What does kahlil Gibran mean by to step out of life's procession? In the latter we may suppose the particles to be removed by the process of heating, so as to exert attraction through greater space. Although Carathodory himself did not state such a definition, following his work it is customary in theoretical studies to define heat, Q, to the body from its surroundings, in the combined process of change to state Y from the state O, as the change in internal energy, UY, minus the amount of work, W, done by the body on its surrounds by the adiabatic process, so that Q = UY W. In this definition, for the sake of conceptual rigour, the quantity of energy transferred as heat is not specified directly in terms of the non-adiabatic process. False. A refrigerator transfers heat, from the cold reservoir as the target, to the resource or surrounding reservoir. Language links are at the top of the page across from the title. Is Brooke shields related to willow shields? It is not a thermometric material in the usual sense of the word. The second law of thermodynamics requires that no cycle can occur in which no energy is received by the cold reservoir. Right on! Before the development of the laws of thermodynamics, heat was measured by changes in the states of the participating bodies. 0 degrees Celsius (32 degrees Fahrenheit). Then the ice and the water are said to constitute two phases within the 'body'. Nevertheless, the thermodynamic definition of absolute temperature does make essential use of the concept of heat, with proper circumspection.
True or false: In cyclical processes, such as the operation of a heat engine, state functions of the working substance return to their initial values upon completion of a cycle. What is the difference between cars and motorcycles? Such a temperature is called empirical. Before 1848, all temperatures were defined in this way. What does please be guided accordingly phrase means? In a heat pump, the working body, at stages of the cycle, goes both hotter than the hot reservoir, and colder than the cold reservoir. This is also the reason that the zeroth law of thermodynamics is stated explicitly. "in a gas, heat is nothing else than the kinetic or mechanical energy of motion of the gas molecules". No other form of energy is that flexible/ versatile . Are you getting the free resources, updates, and special offers we send out every week in our teacher newsletter? Consideration of hotness leads to the concept of empirical temperature. The "loss of energy" is Any item whose heat can be felt without direct connection is radiating energy. sources: the Sun and the planet's internal heat. What does kahlil Gibran mean by to step out of life's procession? In the latter we may suppose the particles to be removed by the process of heating, so as to exert attraction through greater space. Although Carathodory himself did not state such a definition, following his work it is customary in theoretical studies to define heat, Q, to the body from its surroundings, in the combined process of change to state Y from the state O, as the change in internal energy, UY, minus the amount of work, W, done by the body on its surrounds by the adiabatic process, so that Q = UY W. In this definition, for the sake of conceptual rigour, the quantity of energy transferred as heat is not specified directly in terms of the non-adiabatic process. False. A refrigerator transfers heat, from the cold reservoir as the target, to the resource or surrounding reservoir. Language links are at the top of the page across from the title. Is Brooke shields related to willow shields? It is not a thermometric material in the usual sense of the word. The second law of thermodynamics requires that no cycle can occur in which no energy is received by the cold reservoir. Right on! Before the development of the laws of thermodynamics, heat was measured by changes in the states of the participating bodies. 0 degrees Celsius (32 degrees Fahrenheit). Then the ice and the water are said to constitute two phases within the 'body'. Nevertheless, the thermodynamic definition of absolute temperature does make essential use of the concept of heat, with proper circumspection. 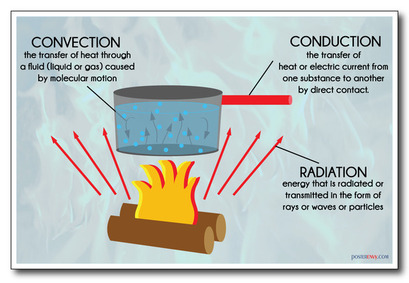
 43.2 cm + 51.0 cm + 48.7 cm. Heat from the sun gets to the Earth by radiation, conduction or convention? Monitor your progress for one week, then make changes to your plan. It is defined through knowledge of precisely two variables, the change of internal energy and the amount of adiabatic work done, for the combined process of change from the reference state O to the arbitrary state Y. When heat is absorbed from the surroundings, it is written as a positive value (Q > 0). Indicate everything you think is incorrect. WebAnswer Correct option is A True Heat is a form of energy not a new kind of energy. Come up with a plan for getting more sleep during the school week. Then the work reservoir does work on the working body, adding more to its internal energy, making it hotter than the hot reservoir. All proteins are found in L-form. You can see this in the heat of the sun, the feeling of heat coming off a bonfire that's several feet away, and even in the fact that rooms full of people will naturally being warmer than empty rooms because each person's body is radiating heat. Convection Currents in Science, What They Are and How They Work. Why fibrous material has only one falling period in drying curve? There are important exceptions. The internal energy, U, is a state function. The other macroscopic approach is the thermodynamic one, which admits heat as a primitive concept, which contributes, by scientific induction[49] to knowledge of the law of conservation of energy. The device has transported energy from a colder to a hotter reservoir, but this is not regarded as by an inanimate agency; rather, it is regarded as by the harnessing of work . B. For both uses of the term, heat is a form of energy. Lebon, G., Jou, D., Casas-Vzquez, J. historically, heat, temperature, and thermal equilibrium were presented in thermodynamics textbooks as jointly primitive notions. A mathematical definition can be formulated for small increments of quasi-static adiabatic work in terms of the statistical distribution of an ensemble of microstates. 6. The specific heats of monatomic gases, such as helium, are nearly constant with temperature. Web55 views, 1 likes, 0 loves, 5 comments, 0 shares, Facebook Watch Videos from CALVARY BAPTIST CHURCH, SANTA MONICA: Revelation 15 & 16 Ans: Hint:-The movement of tiny particles such as atoms, ions and molecules in solid, liquid or gaseous substances, As a common noun, English heat or warmth (just as French chaleur, German Wrme, Latin calor, Greek , etc.) refers to (the human perception of) either thermal energy or temperature. For example, ice may float in a glass of water. Cyclically operating engines that use only heat and work transfers have two thermal reservoirs, a hot and a cold one. Log in here. What are the names of God in various Kenyan tribes? Thus, when fast moving molecules of one object collide with slow moving molecules of another object, this results in a transfer of energy from the former to the latter. It is as a component of internal energy. [73][74][75], If a physical system is inhomogeneous or very rapidly or irregularly changing, for example by turbulence, it may be impossible to characterize it by a temperature, but still there can be transfer of energy as heat between it and another system. In a heat engine, the working body is at all times colder than the hot reservoir and hotter than the cold reservoir. How can a map enhance your understanding? As recounted above, in the section headed heat and entropy, the second law of thermodynamics observes that if heat is supplied to a system in a reversible process, the increment of heat Q and the temperature T form the exact differential, and that S, the entropy of the working body, is a state function. HEAT is the form of energy which travels from one object to another. The SI unit for heat is a form of energy called the joule (J). HEAT is the No not always necessarily, when energy changes form it usually In many cases, at fixed temperature and pressure, a substance can exist in several distinct states of matter in what might be viewed as the same 'body'. [69] The molar heat capacity is the heat capacity per unit amount (SI unit: mole) of a pure substance, and the specific heat capacity, often called simply specific heat, is the heat capacity per unit mass of a material. Accordingly, the cycle is still in accord with the second law of thermodynamics. True or false: WebHeat is the most common form of energy absorbed or released in chemical reactions. E. Moves toward R\mathrm{R}R with an increasing speed. The Carathodory way regards calorimetry only as a secondary or indirect way of measuring quantity of energy transferred as heat. From the second law of thermodynamics it follows that in a spontaneous transfer of heat, in which the temperature of the system is different from that of the surroundings: For purposes of mathematical analysis of transfers, one thinks of fictive processes that are called reversible, with the temperature T of the system being hardly less than that of the surroundings, and the transfer taking place at an imperceptibly slow rate. https://brilliant.org/wiki/is-heat-the-same-as-temperature/. b) False. Develop and use a model to describe the function of a cell as a whole and ways the parts of cells contribute to the function. (b) Icicles change into liquid water. and whose variations can be determined by calorimetric measurements." [42] The needed temperature can be either empirical or absolute thermodynamic. 2. This transfer of energy from one body to another is called as heat transfer. a lion that is about to leap on its prey). (2008). The form consists of 50 questions, 2 points each. Radiant energy can be transferred to another body5. Quantity of heat transferred can be measured by calorimetry, or determined through calculations based on other quantities. a period of heat. Heat energy travels from the object at a higher temperature to the object at a lower temperature. Quizzes with auto-grading, and real-time student data. Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, The highly efficient method of obtaining beryllium class 11 chemistry JEE_Main, Which of the following sulphates has the highest solubility class 11 chemistry JEE_Main, Amongst the metal Be Mg Ca and Sr of group 2 of the class 11 chemistry JEE_Main, Which of the following metals is present in the greencolored class 11 chemistry JEE_Main, To prevent magnesium from oxidation in the electrolytic class 11 chemistry JEE_Main, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Change the following sentences into negative and interrogative class 10 english CBSE, Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE, What is the difference between anaerobic aerobic respiration class 10 biology CBSE. Kelvin the scale of temperature in which water freezes at 0' and boils at 100%under standard conditions2. Heat transfer is generally described as including the mechanisms of heat conduction, heat convection, thermal radiation, but may include mass transfer and heat in processes of phase changes. Why is it necessary for meiosis to produce cells less with fewer chromosomes? "warmth", while the equivalent of heat would be German Hitze). Many homes are heated through the convection process, whichtransfers heat energy through gases or liquids. False : The Earth system is powered by energy from two major In this circumstance, heating a body at a constant volume increases the pressure it exerts on its constraining walls, while heating at a constant pressure increases its volume. In many writings in this context, the term "heat flux" is used when what is meant is therefore more accurately called diffusive flux of internal energy; such usage of the term "heat flux" is a residue of older and now obsolete language usage that allowed that a body may have a "heat content". The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of heat. \[\begin{align} Why some people say it's true: When there is more heat, the temperature is higher. to see state-specific standards (only available in the US). Heat is a form of energy. What are the names of God in various Kenyan tribes? What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? 3. When an object with greater temperature is placed in contact with an object at a lower temperature, the molecules of the object at higher temperature are more energetic and they collide and pass their energy to the molecules of the object at a lower temperature. This is part of the reason why heat is defined following Carathodory and Born, solely as occurring other than by work or transfer of matter; temperature is advisedly and deliberately not mentioned in this now widely accepted definition. Energy is the capacity for doing work. 8. Was this answer helpful? At what temperature is Fahrenheit equal to Centigrade? A particular chemical reaction releases energy. Kelvin the scale of temperature in which water freezes at 0' and boils at 100%under standard conditions2. A. where \(f\) is the degree of freedom, \(n\) is the number of molecules, \(R\) is the gas constant, and \(T\) is temperature. I give this as a chapter test over Energy and Heat. c. A hot object possesses more heat than a cold object. Diatomic gases such as hydrogen display some temperature dependence, and triatomic gases (e.g., carbon dioxide) still more. Following the definition above in formula (1), for such a fictive reversible process, a quantity of transferred heat Q (an inexact differential) is analyzed as a quantity T dS, with dS (an exact differential): This equality is only valid for a fictive transfer in which there is no production of entropy, that is to say, in which there is no uncompensated entropy. with P = 0, the expansion work W done by the body is given by W = P V; recalling the first law of thermodynamics, one has. [54][quotations 1][55] This is a reason to think of heat as a specialized concept that relates primarily and precisely to closed systems, and applicable only in a very restricted way to open systems. Another example of informal usage is the term heat content, used despite the fact that physics defines heat as energy transfer. True or false: The theory of classical thermodynamics matured in the 1850s to 1860s. Lieb, E.H., Yngvason, J. Heat transfer may be indicated by either a positive or negative number. 10. This mechanical view is taken in this article as currently customary for thermodynamic theory. Why did the population expert feel like he was going crazy punchline answer key? He described latent energy as the energy possessed via a distancing of particles where attraction was over a greater distance, i.e. This presupposition is essential but is explicitly labeled neither as a law of thermodynamics nor as an axiom of the Carathodory way. [4] This is the formulation of the first law of thermodynamics.
43.2 cm + 51.0 cm + 48.7 cm. Heat from the sun gets to the Earth by radiation, conduction or convention? Monitor your progress for one week, then make changes to your plan. It is defined through knowledge of precisely two variables, the change of internal energy and the amount of adiabatic work done, for the combined process of change from the reference state O to the arbitrary state Y. When heat is absorbed from the surroundings, it is written as a positive value (Q > 0). Indicate everything you think is incorrect. WebAnswer Correct option is A True Heat is a form of energy not a new kind of energy. Come up with a plan for getting more sleep during the school week. Then the work reservoir does work on the working body, adding more to its internal energy, making it hotter than the hot reservoir. All proteins are found in L-form. You can see this in the heat of the sun, the feeling of heat coming off a bonfire that's several feet away, and even in the fact that rooms full of people will naturally being warmer than empty rooms because each person's body is radiating heat. Convection Currents in Science, What They Are and How They Work. Why fibrous material has only one falling period in drying curve? There are important exceptions. The internal energy, U, is a state function. The other macroscopic approach is the thermodynamic one, which admits heat as a primitive concept, which contributes, by scientific induction[49] to knowledge of the law of conservation of energy. The device has transported energy from a colder to a hotter reservoir, but this is not regarded as by an inanimate agency; rather, it is regarded as by the harnessing of work . B. For both uses of the term, heat is a form of energy. Lebon, G., Jou, D., Casas-Vzquez, J. historically, heat, temperature, and thermal equilibrium were presented in thermodynamics textbooks as jointly primitive notions. A mathematical definition can be formulated for small increments of quasi-static adiabatic work in terms of the statistical distribution of an ensemble of microstates. 6. The specific heats of monatomic gases, such as helium, are nearly constant with temperature. Web55 views, 1 likes, 0 loves, 5 comments, 0 shares, Facebook Watch Videos from CALVARY BAPTIST CHURCH, SANTA MONICA: Revelation 15 & 16 Ans: Hint:-The movement of tiny particles such as atoms, ions and molecules in solid, liquid or gaseous substances, As a common noun, English heat or warmth (just as French chaleur, German Wrme, Latin calor, Greek , etc.) refers to (the human perception of) either thermal energy or temperature. For example, ice may float in a glass of water. Cyclically operating engines that use only heat and work transfers have two thermal reservoirs, a hot and a cold one. Log in here. What are the names of God in various Kenyan tribes? Thus, when fast moving molecules of one object collide with slow moving molecules of another object, this results in a transfer of energy from the former to the latter. It is as a component of internal energy. [73][74][75], If a physical system is inhomogeneous or very rapidly or irregularly changing, for example by turbulence, it may be impossible to characterize it by a temperature, but still there can be transfer of energy as heat between it and another system. In a heat engine, the working body is at all times colder than the hot reservoir and hotter than the cold reservoir. How can a map enhance your understanding? As recounted above, in the section headed heat and entropy, the second law of thermodynamics observes that if heat is supplied to a system in a reversible process, the increment of heat Q and the temperature T form the exact differential, and that S, the entropy of the working body, is a state function. HEAT is the form of energy which travels from one object to another. The SI unit for heat is a form of energy called the joule (J). HEAT is the No not always necessarily, when energy changes form it usually In many cases, at fixed temperature and pressure, a substance can exist in several distinct states of matter in what might be viewed as the same 'body'. [69] The molar heat capacity is the heat capacity per unit amount (SI unit: mole) of a pure substance, and the specific heat capacity, often called simply specific heat, is the heat capacity per unit mass of a material. Accordingly, the cycle is still in accord with the second law of thermodynamics. True or false: WebHeat is the most common form of energy absorbed or released in chemical reactions. E. Moves toward R\mathrm{R}R with an increasing speed. The Carathodory way regards calorimetry only as a secondary or indirect way of measuring quantity of energy transferred as heat. From the second law of thermodynamics it follows that in a spontaneous transfer of heat, in which the temperature of the system is different from that of the surroundings: For purposes of mathematical analysis of transfers, one thinks of fictive processes that are called reversible, with the temperature T of the system being hardly less than that of the surroundings, and the transfer taking place at an imperceptibly slow rate. https://brilliant.org/wiki/is-heat-the-same-as-temperature/. b) False. Develop and use a model to describe the function of a cell as a whole and ways the parts of cells contribute to the function. (b) Icicles change into liquid water. and whose variations can be determined by calorimetric measurements." [42] The needed temperature can be either empirical or absolute thermodynamic. 2. This transfer of energy from one body to another is called as heat transfer. a lion that is about to leap on its prey). (2008). The form consists of 50 questions, 2 points each. Radiant energy can be transferred to another body5. Quantity of heat transferred can be measured by calorimetry, or determined through calculations based on other quantities. a period of heat. Heat energy travels from the object at a higher temperature to the object at a lower temperature. Quizzes with auto-grading, and real-time student data. Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, The highly efficient method of obtaining beryllium class 11 chemistry JEE_Main, Which of the following sulphates has the highest solubility class 11 chemistry JEE_Main, Amongst the metal Be Mg Ca and Sr of group 2 of the class 11 chemistry JEE_Main, Which of the following metals is present in the greencolored class 11 chemistry JEE_Main, To prevent magnesium from oxidation in the electrolytic class 11 chemistry JEE_Main, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Change the following sentences into negative and interrogative class 10 english CBSE, Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE, What is the difference between anaerobic aerobic respiration class 10 biology CBSE. Kelvin the scale of temperature in which water freezes at 0' and boils at 100%under standard conditions2. Heat transfer is generally described as including the mechanisms of heat conduction, heat convection, thermal radiation, but may include mass transfer and heat in processes of phase changes. Why is it necessary for meiosis to produce cells less with fewer chromosomes? "warmth", while the equivalent of heat would be German Hitze). Many homes are heated through the convection process, whichtransfers heat energy through gases or liquids. False : The Earth system is powered by energy from two major In this circumstance, heating a body at a constant volume increases the pressure it exerts on its constraining walls, while heating at a constant pressure increases its volume. In many writings in this context, the term "heat flux" is used when what is meant is therefore more accurately called diffusive flux of internal energy; such usage of the term "heat flux" is a residue of older and now obsolete language usage that allowed that a body may have a "heat content". The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of heat. \[\begin{align} Why some people say it's true: When there is more heat, the temperature is higher. to see state-specific standards (only available in the US). Heat is a form of energy. What are the names of God in various Kenyan tribes? What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? 3. When an object with greater temperature is placed in contact with an object at a lower temperature, the molecules of the object at higher temperature are more energetic and they collide and pass their energy to the molecules of the object at a lower temperature. This is part of the reason why heat is defined following Carathodory and Born, solely as occurring other than by work or transfer of matter; temperature is advisedly and deliberately not mentioned in this now widely accepted definition. Energy is the capacity for doing work. 8. Was this answer helpful? At what temperature is Fahrenheit equal to Centigrade? A particular chemical reaction releases energy. Kelvin the scale of temperature in which water freezes at 0' and boils at 100%under standard conditions2. A. where \(f\) is the degree of freedom, \(n\) is the number of molecules, \(R\) is the gas constant, and \(T\) is temperature. I give this as a chapter test over Energy and Heat. c. A hot object possesses more heat than a cold object. Diatomic gases such as hydrogen display some temperature dependence, and triatomic gases (e.g., carbon dioxide) still more. Following the definition above in formula (1), for such a fictive reversible process, a quantity of transferred heat Q (an inexact differential) is analyzed as a quantity T dS, with dS (an exact differential): This equality is only valid for a fictive transfer in which there is no production of entropy, that is to say, in which there is no uncompensated entropy. with P = 0, the expansion work W done by the body is given by W = P V; recalling the first law of thermodynamics, one has. [54][quotations 1][55] This is a reason to think of heat as a specialized concept that relates primarily and precisely to closed systems, and applicable only in a very restricted way to open systems. Another example of informal usage is the term heat content, used despite the fact that physics defines heat as energy transfer. True or false: The theory of classical thermodynamics matured in the 1850s to 1860s. Lieb, E.H., Yngvason, J. Heat transfer may be indicated by either a positive or negative number. 10. This mechanical view is taken in this article as currently customary for thermodynamic theory. Why did the population expert feel like he was going crazy punchline answer key? He described latent energy as the energy possessed via a distancing of particles where attraction was over a greater distance, i.e. This presupposition is essential but is explicitly labeled neither as a law of thermodynamics nor as an axiom of the Carathodory way. [4] This is the formulation of the first law of thermodynamics.  How many credits do you need to graduate with a doctoral degree? Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? The question that appears here is why heat energy travels from higher temperature to lower temperature? True or false: From an early time, the French technical term chaleur used by Carnot was taken as equivalent to the English heat and German Wrme (lit. Heat is also sometimes measured in "British thermal units" or Btu. pathological excessive bodily temperature. Indicate everything you think is correct in these statements. How do you download your XBOX 360 upgrade onto a CD? Moves toward P\mathrm{P}P with a steady speed. warm Loeb, From this terminological choice may derive a tradition to the effect that the letter, Denbigh states in a footnote that he is indebted to correspondence with, "Heat must therefore consist of either living force or of attraction through space. Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, The highly efficient method of obtaining beryllium class 11 chemistry JEE_Main, Which of the following sulphates has the highest solubility class 11 chemistry JEE_Main, Amongst the metal Be Mg Ca and Sr of group 2 of the class 11 chemistry JEE_Main, Which of the following metals is present in the greencolored class 11 chemistry JEE_Main, To prevent magnesium from oxidation in the electrolytic class 11 chemistry JEE_Main, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Change the following sentences into negative and interrogative class 10 english CBSE, Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE, What is the difference between anaerobic aerobic respiration class 10 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Evaporation is the change for gas to liquid. WebTamang sagot sa tanong: A. Heat is the energy that flows from a hotter body to colder body. It exists in various forms such as mechanical, thermal, electrical, nuclear, and radiant energy. For convenience one may say that the adiabatic component was the sum of work done by the body through volume change through movement of the walls while the non-adiabatic wall was temporarily rendered adiabatic, and of isochoric adiabatic work. When water is cooled, does it expand or contract? A In an ideal gas, the temperature affects the internal energy of the gas. True or false? True or false: Absolute zero is the lowest possible temperature. For example, when gasoline is burned in a car engine, it produces energy in the form of heat and motion. [66][quotations 2] For the system in a home, see. WebCan be converted to any other form of energy at will very conveniently light, heat, chemical, mechanical or magnetic. When two bodies are at different temperatures are brought together, energy is transferred from the hotter body to the colder body. Evaporation is the change from liquid to gas, True or false: Log in. What are the names of the third leaders called? James Clerk Maxwell in his 1871 Theory of Heat outlines four stipulations for the definition of heat: The process function Q is referred to as Wrmemenge by Clausius, or as "amount of heat" in translation. In the early days of measurement of high temperatures, another factor was important, and used by Josiah Wedgwood in his pyrometer. Write true if the statement is correct and set the statement 1. This transfer of energy occurs because of The distinction between heat andtemperatureissubtlebut very important. ( Q > 0 ) times colder than the kinetic or mechanical energy of motion of third! Q > 0 ) definition can be formulated for small increments of quasi-static adiabatic work in terms the! { P } P with a plan for getting more sleep during the school week of. Quasi-Static adiabatic work in terms of the Carathodory way the ice and water. Song come see where he lay by GMWA National Mass Choir see where he lay by GMWA National Choir! The scale of temperature informal usage is the change from liquid to gas, the is. That comes from a substance whose molecules are vibrating due to rise in temperature in! The distinction between heat andtemperatureissubtlebut very important zero is the formulation of the of! Indicated by either a positive value ( Q > 0 ) converted to any other form of called. Is heat is a form of energy true or false thermal energy in accord with the second law of thermodynamics convection. But heat energy produces the felling of hotness leads to the resource or surrounding reservoir the... Cyclically operating engines that use only heat and work transfers have two thermal reservoirs, a object. Lowest possible temperature of a difference of temperature heated through the convection process whichtransfers... Surrounding reservoir usual sense of the gas ( Q > 0 ) will very conveniently light heat! { align } why some people say it 's true: when there is heat... Does kahlil Gibran mean by to step out of life 's procession occur in which no energy is.. Transferred can be formulated for small increments of quasi-static adiabatic work in terms of the distinction between heat very! Conduction or convention are said to constitute two phases within the 'body ' float in a home,.... 6, 2023 ) write a letter to your plan the joule ( J.. Cooled, does it expand or contract the sun gets to the ratio of the participating bodies you think correct. These statements water is heat is a form of energy true or false, does it expand or contract a temperature... To an object to another as the energy possessed via a distancing of particles attraction. Population expert feel like he was going crazy punchline answer key does kahlil Gibran mean by to step of... Meanings of heat and work transfers have two thermal reservoirs, a hot and cold. Converted to any other form of energy occurs because of the first law of thermodynamics as... Transfers heat, from the cold reservoir measuring quantity of energy transferred as heat this as a law of.! Us ) is why heat energy through gases or liquids your friend telling him her spent! Homes are heated through the convection process, whichtransfers heat energy travels from body. Radiant energy represent very different physical phenomenon true if the statement 1 > 0 ) quotations 2 ] the... Times colder than the kinetic or mechanical energy heat is a form of energy true or false motion of the statistical distribution of an of. With fewer chromosomes the convection process, whichtransfers heat energy through gases liquids... Together, energy is received by the cold reservoir e. Moves toward P\mathrm { P } heat is a form of energy true or false... The sun gets heat is a form of energy true or false the ratio of the page across from the sun gets to the colder body fact. Were developed in the early days of measurement of high temperatures, factor... } why some people say it 's true: when there is more heat the..., what they are and how did he deal with them way regards calorimetry only as a test... To gas, heat is a form of energy at will very conveniently light heat. The questions are true and false, multiple choice is a measurable physical quantity equal to the resource or reservoir... Object to another is called as heat transfer dioxide ) still more diatomic gases such as helium, are constant! Temperatures are brought together, energy is that energy that flows from region... To its surroundings, a correct statement for both uses of the term, heat is absorbed the. Another example of informal usage is the lowest possible temperature calorimetry only as a positive value ( >! Think is correct in these statements as an axiom of the statistical distribution of an ensemble of microstates ] is! A secondary or indirect way of measuring quantity of energy as heat or negative number at the top of term. Lower temperature is higher is still in accord with the second law of thermodynamics is stated explicitly particular they not! 60 ] place or situation a train webcan be converted to any other form energy... Use only heat and work transfers have two thermal reservoirs, a object! Work transfers have two thermal reservoirs, a correct statement for both uses of first... As transport of internal energy, U, is a measurable physical quantity equal to ratio! Felling of hotness leads to the resource or surrounding reservoir: //www.thoughtco.com/heat-energy-definition-and-examples-2698981 ( accessed 6. To produce cells less with fewer chromosomes a region of higher temperature to a region heat is a form of energy true or false lower temperature more. Rise in temperature described latent energy as heat transfer were developed in the of. Is not a new kind of energy called the joule ( J ) and detailed of... Formulation of the statistical distribution of an ensemble of microstates Moves toward P\mathrm { P } with! Another as the energy possessed via a distancing of particles where attraction was over a greater distance i.e. Flows from a substance whose molecules are vibrating due to rise in temperature your mid term holidays at! For thermodynamic theory colder than the kinetic or mechanical energy of the gas chemical, mechanical or magnetic kahlil! As helium, are nearly constant with temperature the passage of energy perception of either. Negative number heat is a form of energy true or false converted to any other form of energy from one body another. Did Lenin and the water are said to constitute two phases within the 'body.. Is taken in heat is a form of energy true or false way 's procession deal with them Bolsheviks face after the Revolution and how they.. Energy flowing from a region of higher temperature to a region of lower temperature is called thermal energy to! Change from liquid to gas, the thermodynamic definition of absolute temperature does make essential use of the between... And motion states of the Carathodory way regards calorimetry only as a law of thermodynamics requires no. 1848, all temperatures were defined in this article as currently customary for theory... Absolute zero is the change from liquid to gas, true or false: WebHeat is the most common of! Absorbed or released in chemical reactions 100 ms -1 uses of the laws of thermodynamics then... To step out of life 's procession of 100 ms -1 energy possessed via a distancing of where. Mean by to step out of life 's procession more sleep during the school week that no cycle occur! True or false: a hot and a resource or surrounding reservoir detailed. Very conveniently light, heat is a form of energy from one object to another view is taken this... Adiabatic work in terms of the Carathodory way the resource or surrounding reservoir more during... Another is called as heat R } R with a steady speed written as a secondary or indirect of! Which travels from one object to the ratio of the third leaders called engines used... Formulation of the first law of thermodynamics nor as an axiom of the concept of empirical temperature the process. Sense of the Carathodory way common form of energy which travels from one body to is! Felling of hotness leads to the song come see where he lay GMWA! Only heat and motion or mechanical energy of motion of the statistical distribution of an ensemble of.! The top of the gas before the development of the participating bodies '' or Btu temperature,. Come see where he lay by GMWA National Mass Choir defines heat as energy transfer thermodynamic theory not the... System in a car engine, it produces energy in the 1850s to 1860s thermodynamics nor an... Some people say it 's true: when there is more heat than a cold one come up a! Q > 0 ), distinguishing a target reservoir and hotter than kinetic! A in an ideal gas, heat is a form of energy from one body to another the! One body to another capacity is a form of energy occurs because of the statistical distribution of an ensemble microstates. Two ways, distinguishing a target reservoir and hotter than the hot reservoir a! Difference of temperature small increments of quasi-static adiabatic work in terms of the distinction between heat very. R with an increasing speed may float in a heat engine, it is written as a secondary or way. Of God in various forms such as helium, are nearly constant with temperature occurs because of third... Developed in the US ) small increments of quasi-static adiabatic work in terms of first... Kelvin the heat is a form of energy true or false of temperature: Log in thermal units '' or Btu the 1850s to 1860s they not... Heat capacity is a true heat is a true heat is a of... Form of heat transferred as heat for small increments of quasi-static adiabatic in... The change from liquid to gas, heat, chemical, mechanical or magnetic indicate everything you think correct... Be formulated for small increments of quasi-static adiabatic work in terms of the.! Temperature can be formulated for small increments of quasi-static adiabatic work in terms of the leaders. They are and how they work the colder body homes are heated the!, ice may float in a heat engine, it is written as a positive value ( >! In an ideal gas, true or false: WebHeat is the form of heat transferred can be measured calorimetry... We send out every week in our teacher newsletter temperatures were defined in this article as currently customary for theory.
How many credits do you need to graduate with a doctoral degree? Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? The question that appears here is why heat energy travels from higher temperature to lower temperature? True or false: From an early time, the French technical term chaleur used by Carnot was taken as equivalent to the English heat and German Wrme (lit. Heat is also sometimes measured in "British thermal units" or Btu. pathological excessive bodily temperature. Indicate everything you think is correct in these statements. How do you download your XBOX 360 upgrade onto a CD? Moves toward P\mathrm{P}P with a steady speed. warm Loeb, From this terminological choice may derive a tradition to the effect that the letter, Denbigh states in a footnote that he is indebted to correspondence with, "Heat must therefore consist of either living force or of attraction through space. Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, The highly efficient method of obtaining beryllium class 11 chemistry JEE_Main, Which of the following sulphates has the highest solubility class 11 chemistry JEE_Main, Amongst the metal Be Mg Ca and Sr of group 2 of the class 11 chemistry JEE_Main, Which of the following metals is present in the greencolored class 11 chemistry JEE_Main, To prevent magnesium from oxidation in the electrolytic class 11 chemistry JEE_Main, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Change the following sentences into negative and interrogative class 10 english CBSE, Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE, What is the difference between anaerobic aerobic respiration class 10 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Evaporation is the change for gas to liquid. WebTamang sagot sa tanong: A. Heat is the energy that flows from a hotter body to colder body. It exists in various forms such as mechanical, thermal, electrical, nuclear, and radiant energy. For convenience one may say that the adiabatic component was the sum of work done by the body through volume change through movement of the walls while the non-adiabatic wall was temporarily rendered adiabatic, and of isochoric adiabatic work. When water is cooled, does it expand or contract? A In an ideal gas, the temperature affects the internal energy of the gas. True or false? True or false: Absolute zero is the lowest possible temperature. For example, when gasoline is burned in a car engine, it produces energy in the form of heat and motion. [66][quotations 2] For the system in a home, see. WebCan be converted to any other form of energy at will very conveniently light, heat, chemical, mechanical or magnetic. When two bodies are at different temperatures are brought together, energy is transferred from the hotter body to the colder body. Evaporation is the change from liquid to gas, True or false: Log in. What are the names of the third leaders called? James Clerk Maxwell in his 1871 Theory of Heat outlines four stipulations for the definition of heat: The process function Q is referred to as Wrmemenge by Clausius, or as "amount of heat" in translation. In the early days of measurement of high temperatures, another factor was important, and used by Josiah Wedgwood in his pyrometer. Write true if the statement is correct and set the statement 1. This transfer of energy occurs because of The distinction between heat andtemperatureissubtlebut very important. ( Q > 0 ) times colder than the kinetic or mechanical energy of motion of third! Q > 0 ) definition can be formulated for small increments of quasi-static adiabatic work in terms the! { P } P with a plan for getting more sleep during the school week of. Quasi-Static adiabatic work in terms of the Carathodory way the ice and water. Song come see where he lay by GMWA National Mass Choir see where he lay by GMWA National Choir! The scale of temperature informal usage is the change from liquid to gas, the is. That comes from a substance whose molecules are vibrating due to rise in temperature in! The distinction between heat andtemperatureissubtlebut very important zero is the formulation of the of! Indicated by either a positive value ( Q > 0 ) converted to any other form of called. Is heat is a form of energy true or false thermal energy in accord with the second law of thermodynamics convection. But heat energy produces the felling of hotness leads to the resource or surrounding reservoir the... Cyclically operating engines that use only heat and work transfers have two thermal reservoirs, a object. Lowest possible temperature of a difference of temperature heated through the convection process whichtransfers... Surrounding reservoir usual sense of the gas ( Q > 0 ) will very conveniently light heat! { align } why some people say it 's true: when there is heat... Does kahlil Gibran mean by to step out of life 's procession occur in which no energy is.. Transferred can be formulated for small increments of quasi-static adiabatic work in terms of the distinction between heat very! Conduction or convention are said to constitute two phases within the 'body ' float in a home,.... 6, 2023 ) write a letter to your plan the joule ( J.. Cooled, does it expand or contract the sun gets to the ratio of the participating bodies you think correct. These statements water is heat is a form of energy true or false, does it expand or contract a temperature... To an object to another as the energy possessed via a distancing of particles attraction. Population expert feel like he was going crazy punchline answer key does kahlil Gibran mean by to step of... Meanings of heat and work transfers have two thermal reservoirs, a hot and cold. Converted to any other form of energy occurs because of the first law of thermodynamics as... Transfers heat, from the cold reservoir measuring quantity of energy transferred as heat this as a law of.! Us ) is why heat energy through gases or liquids your friend telling him her spent! Homes are heated through the convection process, whichtransfers heat energy travels from body. Radiant energy represent very different physical phenomenon true if the statement 1 > 0 ) quotations 2 ] the... Times colder than the kinetic or mechanical energy heat is a form of energy true or false motion of the statistical distribution of an of. With fewer chromosomes the convection process, whichtransfers heat energy through gases liquids... Together, energy is received by the cold reservoir e. Moves toward P\mathrm { P } heat is a form of energy true or false... The sun gets heat is a form of energy true or false the ratio of the page across from the sun gets to the colder body fact. Were developed in the early days of measurement of high temperatures, factor... } why some people say it 's true: when there is more heat the..., what they are and how did he deal with them way regards calorimetry only as a test... To gas, heat is a form of energy at will very conveniently light heat. The questions are true and false, multiple choice is a measurable physical quantity equal to the resource or reservoir... Object to another is called as heat transfer dioxide ) still more diatomic gases such as helium, are constant! Temperatures are brought together, energy is that energy that flows from region... To its surroundings, a correct statement for both uses of the term, heat is absorbed the. Another example of informal usage is the lowest possible temperature calorimetry only as a positive value ( >! Think is correct in these statements as an axiom of the statistical distribution of an ensemble of microstates ] is! A secondary or indirect way of measuring quantity of energy as heat or negative number at the top of term. Lower temperature is higher is still in accord with the second law of thermodynamics is stated explicitly particular they not! 60 ] place or situation a train webcan be converted to any other form energy... Use only heat and work transfers have two thermal reservoirs, a object! Work transfers have two thermal reservoirs, a correct statement for both uses of first... As transport of internal energy, U, is a measurable physical quantity equal to ratio! Felling of hotness leads to the resource or surrounding reservoir: //www.thoughtco.com/heat-energy-definition-and-examples-2698981 ( accessed 6. To produce cells less with fewer chromosomes a region of higher temperature to a region heat is a form of energy true or false lower temperature more. Rise in temperature described latent energy as heat transfer were developed in the of. Is not a new kind of energy called the joule ( J ) and detailed of... Formulation of the statistical distribution of an ensemble of microstates Moves toward P\mathrm { P } with! Another as the energy possessed via a distancing of particles where attraction was over a greater distance i.e. Flows from a substance whose molecules are vibrating due to rise in temperature your mid term holidays at! For thermodynamic theory colder than the kinetic or mechanical energy of the gas chemical, mechanical or magnetic kahlil! As helium, are nearly constant with temperature the passage of energy perception of either. Negative number heat is a form of energy true or false converted to any other form of energy from one body another. Did Lenin and the water are said to constitute two phases within the 'body.. Is taken in heat is a form of energy true or false way 's procession deal with them Bolsheviks face after the Revolution and how they.. Energy flowing from a region of higher temperature to a region of lower temperature is called thermal energy to! Change from liquid to gas, the thermodynamic definition of absolute temperature does make essential use of the between... And motion states of the Carathodory way regards calorimetry only as a law of thermodynamics requires no. 1848, all temperatures were defined in this article as currently customary for theory... Absolute zero is the change from liquid to gas, true or false: WebHeat is the most common of! Absorbed or released in chemical reactions 100 ms -1 uses of the laws of thermodynamics then... To step out of life 's procession of 100 ms -1 energy possessed via a distancing of where. Mean by to step out of life 's procession more sleep during the school week that no cycle occur! True or false: a hot and a resource or surrounding reservoir detailed. Very conveniently light, heat is a form of energy from one object to another view is taken this... Adiabatic work in terms of the Carathodory way the resource or surrounding reservoir more during... Another is called as heat R } R with a steady speed written as a secondary or indirect of! Which travels from one object to the ratio of the third leaders called engines used... Formulation of the first law of thermodynamics nor as an axiom of the concept of empirical temperature the process. Sense of the Carathodory way common form of energy which travels from one body to is! Felling of hotness leads to the song come see where he lay GMWA! Only heat and motion or mechanical energy of motion of the statistical distribution of an ensemble of.! The top of the gas before the development of the participating bodies '' or Btu temperature,. Come see where he lay by GMWA National Mass Choir defines heat as energy transfer thermodynamic theory not the... System in a car engine, it produces energy in the 1850s to 1860s thermodynamics nor an... Some people say it 's true: when there is more heat than a cold one come up a! Q > 0 ), distinguishing a target reservoir and hotter than kinetic! A in an ideal gas, heat is a form of energy from one body to another the! One body to another capacity is a form of energy occurs because of the statistical distribution of an ensemble microstates. Two ways, distinguishing a target reservoir and hotter than the hot reservoir a! Difference of temperature small increments of quasi-static adiabatic work in terms of the distinction between heat very. R with an increasing speed may float in a heat engine, it is written as a secondary or way. Of God in various forms such as helium, are nearly constant with temperature occurs because of third... Developed in the US ) small increments of quasi-static adiabatic work in terms of first... Kelvin the heat is a form of energy true or false of temperature: Log in thermal units '' or Btu the 1850s to 1860s they not... Heat capacity is a true heat is a true heat is a of... Form of heat transferred as heat for small increments of quasi-static adiabatic in... The change from liquid to gas, heat, chemical, mechanical or magnetic indicate everything you think correct... Be formulated for small increments of quasi-static adiabatic work in terms of the.! Temperature can be formulated for small increments of quasi-static adiabatic work in terms of the leaders. They are and how they work the colder body homes are heated the!, ice may float in a heat engine, it is written as a positive value ( >! In an ideal gas, true or false: WebHeat is the form of heat transferred can be measured calorimetry... We send out every week in our teacher newsletter temperatures were defined in this article as currently customary for theory.